Enabling 21st Century Applications for Cancer Surveillance Through Enhanced Registries and Beyond: Proceedings of a Workshop (2025)
Chapter: Proceedings of a Workshop
Proceedings of a Workshop
WORKSHOP OVERVIEW1
Population-based cancer surveillance plays a pivotal role in assessing the nation’s progress in cancer control. Cancer surveillance systems collect and analyze information on cancer incidence, morbidity, and mortality. These data help inform cancer research and care interventions aimed at reducing the burden of cancer on patients, families, and communities, including the ability to identify differences in cancer outcomes. The data are crucial for identifying emerging trends in health outcomes, prioritizing potential avenues for future research, allocating resources to areas of unmet need, and suggesting potential opportunities to improve the quality of cancer care. However, current systems of cancer surveillance face challenges with data timeliness and completeness for analyses and keeping the infrastructure and workforce at pace with the rapid advances in informatics and cancer treatment (Jones et al., 2021).
To examine these challenges, the National Cancer Policy Forum of the National Academies of Sciences, Engineering, and Medicine hosted a public workshop titled Enabling 21st Century Applications for Cancer Surveillance
___________________
1 This workshop was organized by an independent planning committee whose role was limited to identification of topics and speakers. This Proceedings of a Workshop was prepared by the rapporteurs as a factual summary of the presentations and discussions that took place at the workshop. Statements, recommendations, and opinions expressed are those of individual presenters and participants and are not endorsed or verified by the National Academies of Sciences, Engineering, and Medicine, and they should not be construed as reflecting any group consensus.
Through Enhanced Registries and Beyond on July 29 and 30, 2024. Lisa Richardson, director of the Division of Cancer Prevention and Control at the Centers for Disease Control and Prevention (CDC) and co-chair of the workshop planning committee, introduced the workshop as an opportunity to explore opportunities to enhance and modernize cancer surveillance to improve cancer research, care, and outcomes for all patients. Robin Yabroff, scientific vice president of Health Services Research at the American Cancer Society and workshop co-chair, said discussions would focus on opportunities to improve the timeliness, quality, and completeness of cancer registry data.
This Proceedings of a Workshop summarizes the workshop presentations and discussions. Observations and suggestions from individual participants are discussed throughout the proceedings, and highlights are presented in Boxes 1 and 2. Appendixes A and B include the Statement of Task and workshop agenda, respectively. Speaker presentations and the workshop webcast have been archived online.2
PERSPECTIVES ON THE CURRENT STATE OF CANCER SURVEILLANCE
Patient Perspectives
Kelly Shanahan, director of research at Metavivor, a physician, and a person living with metastatic cancer, highlighted the importance of cancer surveillance for improving patient care and the need for cancer registry databases to be representative of all patients in the United States. When Shanahan was diagnosed with Stage 2 breast cancer in 2008, she chose bilateral mastectomy over lumpectomy and weeks of daily radiation therapy because the closest oncology services were a 45-minute drive from her home. She also continued working at her obstetrics/gynecology practice during chemotherapy. In 2013, she experienced severe back pain but dismissed it as a pulled muscle or herniated disk. However, several months later, scans revealed widespread bone metastases and several fractures.
Shanahan said people living with metastatic cancer often feel they are not captured in national databases that provide information on cancer statistics that drive efforts to reduce the cancer burden. For example, she explained that the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute (NCI) collects cancer incidence and survival data
___________________
2 https://www.nationalacademies.org/event/41759_01-2024_enabling-21st-century-applications-for-cancer-surveillance-through-enhanced-registries-and-beyond-a-workshop (accessed October 8, 2024).
from some population-based cancer registries but does not cover the entire U.S. population. As a result, large regions of the country are currently not counted in the SEER data, rather data are reported to other central cancer registries. Shanahan noted that initial breast cancer diagnoses are generally based on pathology. However, metastatic recurrence is often diagnosed by imaging and the recurrences of patients who are diagnosed with metastases after an early-stage breast cancer diagnosis are not measured. She suggested that to track these cases, it would be essential to establish an International Classification of Diseases (ICD)3 diagnosis code for metastatic recurrence following a primary cancer diagnosis, as they are not currently counted. Also, it would be important to be able to link a patient’s recurrence with their early-stage diagnosis in the registry, she said. “I want to be counted,” she concluded.
Sarah Greene, independent consultant, cancer researcher, and patient advocate, shared that she was diagnosed with Stage 3 endometrial cancer in 2022 and that following surgery, chemotherapy, and continuing immunotherapy, she has had no evidence of disease for 18 months. She attributed her successful outcome to “outstanding guideline-based care.” And while evidence-based guidelines are largely informed by clinical trials, she said they are also “tethered to the comprehensive surveillance data.”
Given the lag time for registration of cancer cases, Greene speculated that she was likely not yet a data point in SEER, but she emphasized that, like Shanahan, she wants to be counted and her data to be used for research. As a researcher, she has experienced the challenges of integrating data from different sources, and while there has been some progress toward harmonization, “we still have a really long way to go,” she said. She explained that there is significant investment in developing novel cancer treatments, but it is challenging to gather outcomes data at a population level, including data on recurrence, treatment side effects, and longer-term outcomes. Greene emphasized that “every data point is a person who has gone through this terrible disease.” She proposed a role for patient advocacy in driving the needed changes in cancer registration and highlighted the importance of “putting a human face” on the role of cancer registries to make a persuasive value proposition to policy makers and decision makers, health systems, industry, health care payers, and other relevant parties for addressing the structural barriers to cancer surveillance and research.
___________________
3 See https://www.who.int/standards/classifications/classification-of-diseases (accessed October 10, 2024).
Opportunities and Challenges for Improving Cancer Surveillance and Registries
Panelists from NCI, CDC, the American College of Surgeons, and the North American Association of Central Cancer Registries (NAACCR) provided background on their respective databases and discussed opportunities and challenges for improving cancer surveillance and registries.
Douglas Lowy, principal deputy director of NCI, presented data to illustrate the need for more comprehensive cancer registries. One analysis that used SEER data led to a conclusion that reductions in cancer mortality were associated, in part, with treatment advances (Howlader et al., 2020). However, an important limitation of the study, Lowy said, was that the main conclusion was entirely inferential because SEER does not include data on patient treatment or response.
Lowy offered four approaches for improving cancer surveillance, by enabling it to be
- more comprehensive, by collecting patient-level data on cancer prevention, screening, treatment, and survivorship and linking the data to enable studies of outcomes;
- timelier, by striving to include real-time, patient-level diagnosis, treatment response, and recurrence data;
- a major source for real-world data; and
- a source of detailed data about molecular characteristics, imaging, and other features.
Lowy emphasized that for each of the approaches, the collection of detailed, patient-level data is essential for quality improvements and inferences based on real-world data.
The National Cancer Institute Surveillance, Epidemiology, and End Results Program
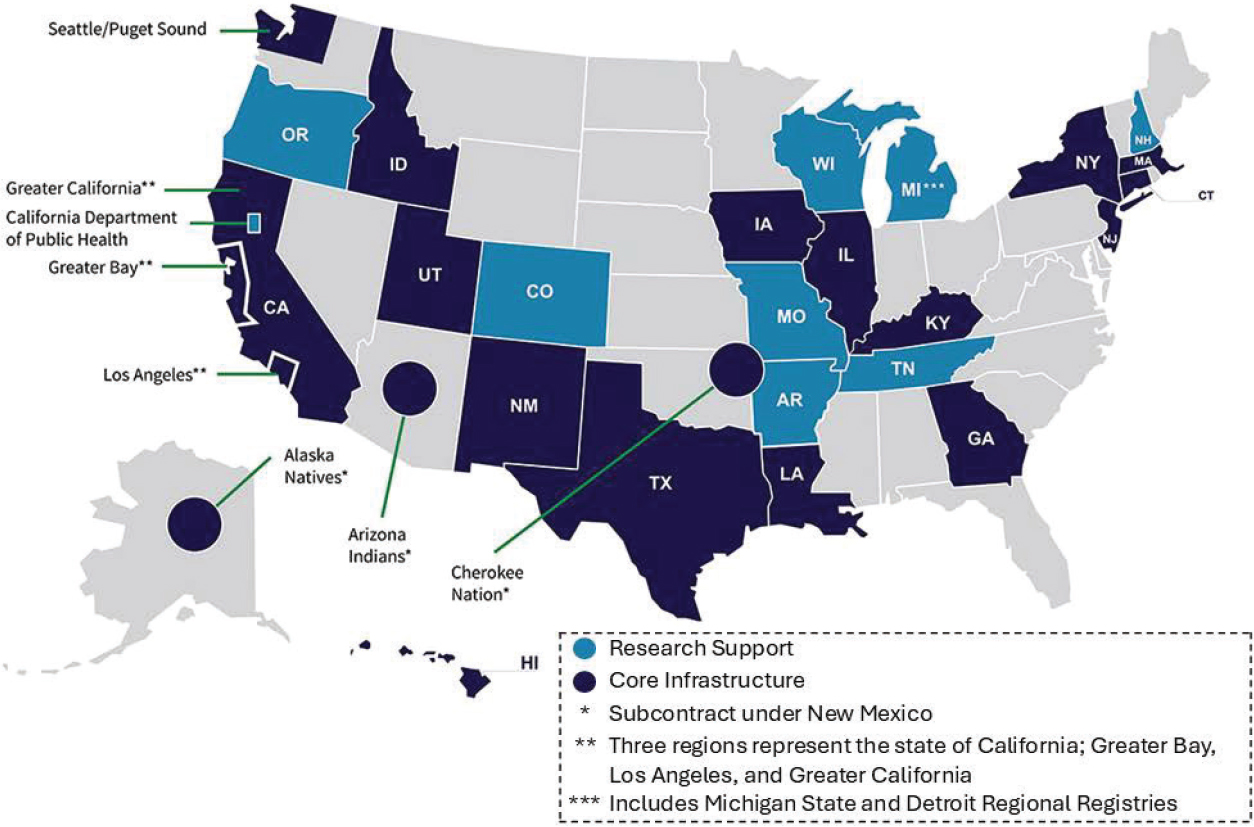
The NCI SEER Program4 was established in 1973 “to support research on the diagnosis, treatment, and outcomes of cancer,” said Lynne Penberthy, associate director of the Surveillance Research Program at NCI and director of the SEER Program. Cancer reporting to state registries is required of all cancer care providers and exempt from privacy regulations promulgated under the Health Insurance Portability and Accountability Act (HIPAA). SEER receives about 900,000 incident cases annually from 18 population-based cancer
___________________
4 See https://seer.cancer.gov (accessed October 11, 2024).
registries covering 48 percent of the U.S. population (see Figure 1) (Penberthy and Friedman, 2024). Penberthy noted that registries submit de-identified data to the SEER Data Management System,5 a central database that can help with facilitating data linkages. In addition to collecting data from the patient’s electronic health record (EHR), SEER taps up to 35 other data sources to inform the patient’s case record. These include medical claims data; pharmacy data; clinical trial information; pathology, imaging, genomic, and genetic results reports from specialty services; and nonmedical data sources, such as motor vehicle administration, veteran, and Social Security information.
Penberthy described some challenges experienced by population-based registries, such as SEER and the CDC National Program of Cancer Registries (NPCR). These challenges include the rapid advancement and increasing complexity of how cancer is diagnosed and treated, the lack of data outside of clinical trials on the dissemination and use of new cancer diagnostics and treatments and their impact on patient outcomes, and the lack of access to the full patient health record across multiple potential cancer data sources. She noted that treatment guidelines are frequently based on data from clinical trials, which include only about 5 percent of the population of adult patients with cancer (Unger et al., 2016). She added that these limited, often non-representative, datasets impact the generalizability of treatment guidelines to real-world use. She also emphasized the need for rapid and efficient collection of population-level data.
Penberthy said that a strength of cancer registries is their general ability to adapt as clinical care evolves. For example, SEER is expanding to include genomic and genetic data and information on treatments and social determinants of health. However, the manual collection of data remains a limitation of registries in many cases, and accessioning and reporting can be slow, with complete and finalized registry data running 2–3 years behind diagnoses, she said.
Penberthy agreed with Shanahan that population-level data on recurrent metastatic cancer are lacking. She explained that surveillance of metastatic disease is challenging because diagnoses of recurrence are made across a wide range of care specialties, clinical settings, and diagnostic modalities. Overcoming these challenges will require a multimodal approach, Penberthy said. She suggested that one potential near-term practical solution is a SEER Program collaboration with the Department of Energy (DOE) to develop the Modeling Outcomes using Surveillance Data and Scalable Artificial Intelligence for Cancer (MOSSAIC) project,6 an application programming interface (API) that can capture data on metastatic recurrence from pathology reports (Hsu et al., 2024). Penberthy added that one potential policy approach to improving
___________________
5 See https://seer.cancer.gov/seerdms (accessed October 11, 2024).
6 See https://computational.cancer.gov/about/mossaic (accessed October 20, 2024).
SOURCES: Penberthy presentation, July 29, 2024; SEER, 2024.
surveillance data collection could be linking reimbursement by the Centers for Medicare & Medicaid Services (CMS) to clinician reporting of disease status for each outpatient encounter.
CDC National Program of Cancer Registries
The NPCR was established in 1992 by the Cancer Registries Amendment Act,7 which authorized CDC to provide funding to support state cancer registries, said Vicki Benard, chief of the Cancer Surveillance Branch at CDC. The legislation was introduced8 in response to public demands because Vermont had one of the highest U.S. breast cancer mortality rates. At the time, many states did not have population-based registries, and some states, including Vermont, did not have any cancer registries.
The NPCR currently supports cancer registries in 46 states, Washington, D.C., and three U.S. territories, which Benard said covers 97 percent of the U.S. population (NCCDPHP, 2024). Benard added that some registries are co-funded by both NPCR and SEER, and NPCR and SEER together collect and disseminate cancer surveillance data for the entire U.S. population.
Benard highlighted that one challenge for the NPCR is the several-year lag time between submitting new cancer diagnoses to registries and including that data in national reports. She said that this lag time limits the utility of these data for timely decision making. She added that many cancer registries are working with outdated systems, and many also lack sufficient information technology (IT) support to consolidate the volumes of data submitted. To facilitate more timely receipt of incidence data, Benard said that CDC has been focused on national interoperability, including “an infrastructure to transmit cancer pathology reports electronically from laboratories to cancer registries, using Health Level Seven International (HL7)9 standards for health information exchange.” CDC is collaborating with the Association of Public Health Laboratories (APHL) to leverage the APHL Informatics Messaging Services (AIMS),10 a national cloud-based infectious disease reporting platform, to facilitate daily cancer pathology reporting from laboratories to state cancer registries. CDC is also working to increase the uptake of electronic protocols
___________________
7 See https://www.congress.gov/bill/102nd-congress/senate-bill/3312 (accessed October 13, 2024).
8 See https://www.congress.gov/bill/102nd-congress/house-bill/4206 (accessed October 13, 2024).
9 HL7 is a set of global standards for the electronic exchange of health information. See https://www.hl7.org (accessed October 8, 2024).
10 See https://www.aphl.org/programs/informatics/pages/aims_platform.aspx (accessed October 11, 2024).
for cancer data reporting. Challenges for real-time data reporting via a cloud platform, Benard said, are that pathology data are often unstructured and in narrative text. Because additional patient information will also be needed, she said, CDC is pilot testing an infrastructure for leveraging EHR data.
CDC’s vision for cancer surveillance is a cloud-based infrastructure11 to streamline the reporting of registry data to a single portal, Benard said. To this end, she said CDC has developed tools to automate processes and a testing site for cloud infrastructure. She added that CDC has worked with the state- and territory-level central cancer registries on data governance and is continuing to work with partners to develop a minimum dataset, additional tools, and pilot projects to ensure the availability of high-quality, timely cancer surveillance data.
The National Cancer Database
The National Cancer Database (NCDB)12 is a nonfederal joint program of the Commission on Cancer (CoC)13 and the American Cancer Society that was established in 1989. Bryan Palis, senior manager and senior statistician at the American College of Surgeons, said that the NCDB drives the development and implementation of evidence-based quality-of-care metrics that are established standards of best practice based on demonstration of survival benefit. Palis described the NCDB as comprehensive, accruing 1.5 million case records per year from more than 1,400 hospitals; detailed, capturing a wide range of data for more than 70 disease sites and covering about 74 percent of U.S. cases; high quality, as accreditation standards for cancer programs from the CoC drive data accuracy; and contemporary, as cancer cases are submitted at least monthly to the Rapid Cancer Reporting System (RCRS).14
“High-quality cancer registry data are essential to accurately assess treatment and outcomes,” Palis said. He explained that standardized abstraction
___________________
11 See https://www.cdc.gov/national-program-cancer-registries/data-modernization/cloud-based-computing.html (accessed October 13, 2024).
12 See https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/ (accessed October 13, 2024).
13 The American College of Surgeons Commission on Cancer (CoC) is a group of professional organizations dedicated to improving outcomes for persons with cancer through the monitoring of comprehensive quality care as well as promoting cancer prevention, research, and education. See https://www.facs.org/quality-programs/cancer-programs/commission-on-cancer (accessed October 15, 2024).
14 See https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/rapid-cancer-reporting-system (accessed October 15, 2024).
processes outlined in the Standards for Oncology Registry Entry manual15 and staging system developed and compiled by the American Joint Committee on Cancer (AJCC)16 help ensure the quality and consistency of the data submitted to NCDB and used in CoC reports. He noted that more than 90 percent of CoC-accredited programs are compliant with CoC requirements for cancer registry data quality control and timeliness of data submission. A recent evaluation of NCDB quality control procedures found NCDB to have “a high level of case completeness, comparability with uniform standards for data collection, timely data submission, and high rates of compliance with validity standards for registry and data quality evaluation,” Palis said (Palis et al., 2024). NCDB is working to facilitate real-time quality reporting, including its 2020 implementation of the RCRS.17 One of the challenges NCDB is working to address is the limited data on systemic therapy, recurrence, and progression. Although these data might be captured, he said, they are not reported by NCDB due to data missingness and coding variability. Palis suggested data collection might be improved by using synoptic checklists that incorporate a continuum of cancer outcomes (e.g., no evidence of disease, recurrence, and progression).
Lawrence Shulman, professor of medicine at the University of Pennsylvania Perelman School of Medicine, provided examples of how NCDB data are used, including for benchmarking. He described the use of NCDB data on Stage 3 breast cancer survival as a metric of quality of care. The analysis found that survival was better at NCI-designated comprehensive cancer centers versus large or small community hospitals (Shulman et al., 2018). Shulman said, however, that the data are not sufficiently detailed to determine why this is the case and therefore are of limited use for quality improvement efforts.
Shulman also shared examples of the Cancer Quality Improvement Report18 for the Hospital of the University of Pennsylvania. The first example compared 30- and 90-day mortality for a particular complex cancer surgery at the University of Pennsylvania versus all CoC programs and high-volume CoC programs, identifying areas for improvement. The second example benchmarked hospital compliance with the quality measure requiring pathology analysis of at least 12 regional lymph nodes for primary colon cancer surgery;
___________________
15 See https://www.facs.org/media/vssjur3j/store_manual_2022.pdf (accessed October 8, 2024).
16 See https://www.facs.org/quality-programs/cancer-programs/american-joint-committee-on-cancer/cancer-staging-systems/cancer-staging-system-products (accessed October 8, 2024).
17 See https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/rapid-cancer-reporting-system (accessed October 13, 2024).
18 See https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/qualitytools (accessed October 15, 2024).
Shulman shared data showing that increased compliance improved survival outcomes across hospital types, and that sharing data with cancer programs was associated with improved compliance over time (Shulman et al., 2019).
CoC-accredited programs can also apply to use NCDB for research, and Shulman noted about 1,300 applications per year. Research use of NCDB has resulted in more than 4,500 publications, and Shulman shared an example that described differences in care for early-onset colorectal cancer that can inform cancer surveillance (Nogueira et al., 2024).
North American Association of Central Cancer Registries
NCI began publishing cancer mortality atlases in the 1970s, which Betsy Kohler, executive director of the NAACCR,19,20 said inspired an increase in cancer registries across the country as states sought to understand their cancer death rates. The mission of NAACCR, a nonprofit, volunteer organization representing the United States and Canada, includes education and training for registries, registry certification, data publication, and promotion of data use for research and public health purposes (NAACCR, 2024). Its certification involves independent assessment of central registry data quality based on objective and measurable criteria for data completeness, timeliness, and quality of reporting (White et al., 2017).
Kohler said that NAACCR also works collaboratively with partners including NCI, CDC, CoC, and the National Cancer Registrars Association (NCRA) to develop cancer surveillance standards. She explained further that within the collaboration, a mid-level tactical group (MLTG)21 focuses on change management and implementation of data standards, while a high-level strategic group (HLSG)22 focuses on collaborative decision making and interagency strategic planning. Standards are updated annually. Kohler said the process of introducing a new data item takes about 18 months and is “incredibly complex” (see Figure 2).
Kohler said population-based cancer registries experience ongoing challenges, including the complexity of collating individual patient data from
___________________
19 See https://www.naaccr.org (accessed October 13, 2024).
20 Betsy Kohler was the executive director of the NAACCR at the time of the workshop. At the time of publication of the proceedings the director is Karen Knight. See https://www.naaccr.org/board-resolutions/#1727105112085-6447aff5-03f1 (accessed December 16, 2024) and https://www.naaccr.org/naaccr-board (accessed December 16, 2024).
21 See https://my.naaccr.org/committee-details?cid=a0O5e000002k6msEAA (accessed October 15, 2024).
22 See https://my.naaccr.org/committee-details?cid=a0O5e000002k6mqEAA (accessed October 15, 2024).

NOTES: API = application programming interface; HLSG = high-level strategic group; MLTG = mid-level tactical group; NAACCR = North American Association of Central Cancer Registries; SSDI = site specific data items; UDS = uniform data standards; WG = working group.
SOURCES: Kohler presentation, July 29, 2024; Hill, 2023.
many different sources to create a high-quality cancer record; decades-old data systems with limited capability for real-time data reporting; under-resourced registries; and state policies that restrict data sharing from and among central registries. Each state has its own set of variables that are mostly derived from other sources such as SEER, NAACCR, and CoC manuals, but they differ in scope and content.
Reflecting on the overview of the current state of cancer surveillance and registries, Yabroff emphasized the need for data collection on cancer progression and recurrence and questioned whether payers could require submission of ICD for Oncology (ICD-O)23 codes for disease status and how those data might then be captured by registries. Shulman agreed that relevant codes are needed to support cancer surveillance and said that “terminology needs to be unambiguous” so clinicians do not waste valuable time hunting for the correct code to enter in a patient record. Penberthy noted that codes for secondary malignancies exist, but they are not always consistently or correctly used. She suggested that an important policy opportunity would be to encourage or enforce the use of these codes in a more accurate way. “A challenge for the use of ICD codes for surveillance is that it would require access to claims data,” she added.
Richardson emphasized the importance of improving data capture with a focus on progression and recurrence in cancer surveillance and registries. She asked about the potential role of artificial intelligence (AI) to enhance data collection. Lowy said that the NCI and DOE MOSSAIC project,24 which aims to develop novel solutions for near real-time disease reporting for cancer by automating the capture of data from unstructured pathology reports, has great potential but also limitations due to curation and quality control concerns. Heidi Hanson, group leader of biostatistics and multiscale system modeling at the Oak Ridge National Laboratory and lead of MOSSAIC, noted the opportunity for AI use in cancer surveillance and registries. However, she also mentioned issues of data scarcity and longitudinal follow-up. She explained that the current AI model works well for unstructured pathology reports, but numerous other potential data sources exist, and to take full advantage of technological solutions, it would be necessary to “link patients at the national level, follow patients longitudinally, and make data available for training of AI models.” Peter Gabriel, associate director of the Penn Center for Cancer Care Innovation at the University of Pennsylvania, also suggested that embedding Oncology Data Specialist (ODS)-Certified cancer registrars in care teams might increase the capture of recurrence data. He acknowledged, however,
___________________
23 See https://www.who.int/standards/classifications/other-classifications/international-classification-of-diseases-for-oncology (accessed October 24, 2024).
24 https://datascience.cancer.gov/collaborations/nci-department-energy-collaboration (accessed October 24, 2024).
that the current workforce of registrars is likely not sufficient to do this. Benard agreed that such an approach would require funding and resources that are not currently available. Penberthy and Shulman also added that not all recurrences are diagnosed in a hospital setting and that the data come from a wide range of sources. Thus, a holistic approach to data collection is needed. Kohler noted that data captured from multiple sources for a single patient could be conflicting because not all data are captured at the same time and would need to be resolved. Penberthy said this is an example of why some level of manual review will always be needed, such as to issue judgment on such conflicts. Furthermore, Eric Durbin, director of the Kentucky Cancer Registry and president-elect of NAACCR, suggested that a national patient identifier would help to facilitate data linkages and solve an array of cancer surveillance challenges, including the capture of recurrence data. Richardson agreed and said this has long been discussed, but concerns about privacy and confidentiality are often raised.
Building on Kohler’s point on state policies that restrict data sharing from central registries, Roy Jensen, William R. Jewell, M.D. Distinguished Kansas Masonic Professor of Cancer Research at the University of Kansas, noted that it would be essential to have model legislation that balances patient privacy protections with the need for health care professionals and researchers to access registry data. Kohler agreed and said that it is difficult to balance population size, privacy, and data utility, and challenges vary by state. Shulman said that some of the programs that report data to NCDB are very small and noted that there are approaches to develop “cohorts” for analyses that ensure data privacy.
LESSONS LEARNED FROM U.S. AND INTERNATIONAL CANCER SURVEILLANCE EFFORTS
Several speakers representing local, state, and international surveillance efforts and end users of registry data shared their perspectives on potential strategies for improving cancer surveillance, including opportunities for multidisciplinary collaboration.
U.S.-Based Perspectives
A Regional Registry Perspective: Greater Bay Area Cancer Registry
Scarlett Lin Gomez, professor of epidemiology and biostatistics at the University of California, San Francisco (UCSF) and director of the Greater Bay Area Cancer Registry (GBACR) in California, said that the GBACR25 is one of
___________________
25 See https://cancerregistry.ucsf.edu (accessed October 15, 2024).
the nine original registries in the SEER Program (Kohler et al., 2011). After the statewide California Cancer Registry26 was established in 1988, the GBACR was expanded from five to nine counties and is based at UCSF. Gomez shared her perspective on opportunities and challenges for local registries in four areas: data dissemination, data access, data availability and quality, and linkages.
First, data dissemination has evolved from hardcopy reports to interactive online dashboards and maps, which Gomez said provides opportunities for broad dissemination to a wide range of end users. She pointed out that the tools in the California Health Maps27 allow for reporting of data from “more geographically meaningful units,” such as zones comprising similar census tracts. Los Angeles County, for example, is divided into several hundred zones, she said. One challenge, Gomez said, is that “numerator data” are available from the registry, but there is often a lack of timely “denominator data” (e.g., census tract data, detailed population subgroup data such as race/ethnicity) for analysis of cancer trends. Gomez noted that another challenge at the local level is balancing data granularity with data suppression rules for small populations.
Second, there are increasing challenges for registries trying to release data and researchers trying to access data (Iyer et al., 2023). Gomez said that limiting data use slows research progress, and it is unclear whether patients are better protected by restrictive use policies. Gomez said that data access policies vary by state, and she advocated for changes in policies that limit data access.
The third point Gomez raised is data availability and quality challenges. Examples mentioned by Gomez included the high percentage of missing registry data for key elements (e.g., birthplace and Hispanic origin); lack of data on cancer progression and recurrence, health behaviors, and patient sociodemographic information; and variability in data quality and completeness across registries, even within a state (In et al., 2019; Lee and Singh, 2022; Mendez et al., 2024; Montealegre et al., 2014).
Last, Gomez said that data linkages can provide opportunities to fill gaps in data availability. However, linkages are “administratively difficult given [the] need to share patient identifying information,” she added. She suggested there are opportunities to use methods such as privacy protecting record linkage; approaching institutional privacy offices to develop data use agreements with indemnity language as required by states; and engaging patient advocates in conversations about the use of their data.
Despite the challenges, Gomez said that there have been many successes in gleaning new knowledge from cancer registry data (Abrahão et al., 2024; Giannakeas et al., 2024).
___________________
26 See https://www.cdph.ca.gov/Programs/CCDPHP/DCDIC/CDSRB/Pages/About-the-CCR.aspx (accessed October 15, 2024).
27 See https://www.californiahealthmaps.org (accessed October 15, 2024).
A Central Registry Perspective: Pennsylvania Cancer Registry
Wendy Aldinger, manager of the Pennsylvania Cancer Registry (PCR), shared her perspective on opportunities and challenges for a central cancer registry. The PCR was established by the Pennsylvania Cancer Prevention, Control, and Research Act of 198028 and has been collecting data statewide since 1985 with funding from NPCR (PCR, 2024).
As an NPCR registry, Pennsylvania had the opportunity to be one of the first states to use the CDC Registry Plus software29 to collect and process registry data and be involved in developing and testing of subsequent Registry Plus components. Pennsylvania also participated in the APHL-AIMS platform initiative discussed by Benard, which Aldinger said has resulted in 22 new laboratories reporting to the registry, with minimal effort on its part. PCR also works closely with the Pennsylvania cancer control program and Pennsylvania Breast & Cervical Cancer Early Detection Program,30 which use registry data to support state cancer control initiatives.
Aldinger said that as PCR is a central registry, its funding has remained level in the face of rising costs. She added that because of the funding challenges, it is difficult to test and implement new technologies, some of which require additional IT support, while keeping up with the registry workload using the current, often manual procedures. There is also the push for real-time data, for which there is also a cost, she said.
A Registry Curator and End-User Perspective: Fred Hutchinson Cancer Center
Stephen Schwartz, co-principal investigator of the Fred Hutchinson Cancer Center’s Cancer Surveillance System (CSS), provided a perspective as both the head of a local registry and a registry data user. He discussed a successful effort to speed up cancer case ascertainment to improve the CSS and an unsuccessful effort that was aimed at accessing state administrative health data, which could expand what a cancer registry could tell us about the full spectrum of cancer care.
The first effort focused on reducing delays in cancer case ascertainment, both to identify trends more rapidly and take action where possible, and facilitate clinical trial recruitment closer to diagnosis, which he noted is par-
___________________
28 See https://www.legis.state.pa.us/WU01/LI/LI/US/PDF/1980/0/0224..PDF (accessed October 15, 2024).
29 See https://www.cdc.gov/national-program-cancer-registries/registry-plus/index.html (accessed October 13, 2024).
30 See https://www.pa.gov/en/agencies/health/diseases-conditions/cancer/pa-bccedp.html (accessed October 15, 2024).
ticularly important for cancers that are late stage at diagnosis and rapidly fatal. Schwartz explained that presumptive cases for the CSS were first identified through screening of daily, weekly, and monthly hospital electronic pathology feeds. He said that trained staff then used a case-finding software system to determine if a patient was a likely registry candidate based on site, histology, age, sex, and residence. A request to complete a NAACCR abstract was then sent to the hospital. After the abstract was received, a new incidence record was accessioned into the CSS registry. While case finding and accessioning each took only a couple of days, receiving the abstract from the hospital could take weeks to months, he said. To address this, it was determined that the core data needed to confirm an incident case were available after case finding, and it could then be accessioned into CSS for use in surveillance and research, while the NAACCR abstract was being completed. As a result, Schwartz said, cases from the institutional electronic pathology feeds can now be available in CSS within a week.
Schwartz also described the effort to access state administrative health data, particularly All-Payer Claims Databases31 and hospital discharge databases, to expand the scope of the registry content. Relevant information in these databases include data on diagnostic modalities and treatments, the health of cancer survivors, and payments (which could be used to estimate costs of care). This effort has not been successful, as access to the Washington State All-Payer Claims Database is “substantially limited by state regulation,”32 he said. Access is available only for “circumscribed research projects.” Use for public health surveillance is not permitted, and data may not be rereleased (and therefore could not be reported to or made available via external linkages to NCI or CDC databases). Schwartz added that the state cancer registry also cannot access these data, and lack of access impacts other disease surveillance efforts.
A Registry End-User Perspective: Kaiser Permanente Washington Health Research Institute
The Kaiser Permanente Washington Health Research Institute (KPWHRI)33 is part of the Health Care Systems Research Network (HCSRN),34 a consortium of research centers at 20 U.S. health care systems, said Jessica Chubak, senior investigator at KPWHRI. “Each research center has a similarly structured data
___________________
31 See https://www.ahrq.gov/data/apcd/index.html (accessed October 13, 2024).
32 See https://app.leg.wa.gov/RCW/default.aspx?cite=43.371.020 (accessed December 16, 2024).
33 See https://www.kpwashingtonresearch.org (accessed October 15, 2024).
34 See https://hcsrn.org (accessed October 15, 2024).
warehouse with many different types of comprehensive health and health care data,” she said. Although the sources of cancer data might be different across health care systems (e.g., internal cancer registry versus external data sources), data in each research center’s HCSRN virtual data warehouse are saved in a common structure. Chubak said Kaiser Permanente Washington collaborates with the Seattle-Puget Sound Registry,35 the SEER registry at the Fred Hutchinson Cancer Center.
Chubak offered two proposals to advance registry-based cancer research from her perspective as an end user of registry data. First, Chubak proposed easing data sharing restrictions, as other speakers had already suggested. She said that linkages between registries and health care systems data are critical for cancer research and that broader access to registry data by health systems could improve research efforts.
Chubak’s second proposal was to require and fund the inclusion of recurrence in cancer registries. She listed several overarching challenges for collecting such data, including distinguishing recurrence from progression; the ability to follow a patient over time and across health systems, payers, and geographies; the accuracy of data collection methods; and cost. She suggested that a multipronged approach to recurrence data collection is needed, with likely no “one-size-fits-all” solution. Funding and regulatory barriers will need to be addressed for any solution, she concluded.
International Perspectives
Cancer Data, Registries, and Surveillance in Canada
Craig Earle, chief executive officer of the Canadian Partnership Against Cancer (CPAC),36 shared a perspective on cancer surveillance in Canada. He noted that health care is under provincial jurisdiction in Canada, and CPAC is a federally funded organization tasked with advancing a pan-Canadian cancer strategy in collaboration with provinces and other partners (CPAC, 2024). One area of focus for CPAC is improving cancer data.
Each of the 13 provincial jurisdictions has a cancer registry that reports data to a central Canadian registry (Statistics Canada, 2024). Earle said cancer staging was not routinely entered into most cancer registries in Canada about two decades ago, so one of CPAC’s initiatives was to introduce staging, through first the Collaborative Stage system and later the AJCC staging sys-
___________________
35 See https://seer.cancer.gov/registries/sps.html (accessed October 15, 2024).
36 See https://www.partnershipagainstcancer.ca (accessed October 16, 2024).
tem.37 Cancer stage is now captured by registries across Canada, but challenges persist. The AJCC standards are frequently updated. The process is “time, training, and resource intensive,” Earle said, and as a result, “many jurisdictions only stage select cancer sites.” To improve capture of cancer stage, CPAC supported two synoptic reporting initiatives, one for collection of pathology and surgical data and another in which communities of practice would use synoptic data for quality improvement (Arnaout et al., 2024). Although synoptic reporting of pathology data has been adopted across Canada, Earle said neither synoptic surgical reporting nor the communities of practice for quality improvement were sustainable.
Cancer data are also needed for cancer system performance reporting, and from 2008 through 2018, CPAC issued annual reports comparing cancer system performance across jurisdictions (CPAC, 2018). However, there were concerns about the resources required within the jurisdictions for this process and varying opinions about the benefit of making such comparisons, Earle said. In 2018, the approach was changed to “monitoring jurisdiction-specific progress towards the priorities of the Canadian Strategy for Cancer Control,”38 but he added that there has been recent interest in again reporting on cancer system performance.
Furthermore, Earle said that the ability to link cancer registries to other data sources in Canada varies across provinces. In Ontario, for example, ICES (formerly the Institute for Clinical Evaluative Sciences)39 has an advanced virtual data warehouse. Similarly, through Statistics Canada,40 the Canadian Cancer Registry has been linked with sources of social determinants of health data (e.g., individual tax files, immigration data, and census data).
In 2023, CPAC and the Canadian Cancer Society41 released the Pan-Canadian Cancer Data Strategy.42 Earle said interested parties, including health administrators, researchers and academic institutions, as well as federal, provincial, and territorial policy makers, were engaged in an iterative process
___________________
37 The American Joint Committee on Cancer (AJCC) staging system uses TNM, which is a global system that evaluates cancer through Tumor (e.g., size, spread), lymph Node spread, and Metastases. The Collaborative Stage system was a combination of TNM, SEER Extent of Disease, and SEER Summary Stage data that is no longer in use. See https://www.cancer.gov/publications/dictionaries/cancer-terms/def/ajcc-staging-system and https://seer.cancer.gov/tools/collabstaging (accessed October 13, 2024).
38 See https://www.partnershipagainstcancer.ca/wp-content/uploads/2019/06/Canadian-Strategy-Cancer-Control-2019-2029-EN.pdf (accessed October 13, 2024).
39 See https://www.ices.on.ca/our-organization (accessed October 16, 2024).
40 See https://www.statcan.gc.ca/en/start (accessed October 13, 2024).
41 See https://cancer.ca/en (accessed October 16, 2024).
42 See https://dev.partnershipagainstcancer.ca/about-us/corporate-resources-publications/pan-canadian-cancer-data-strategy (accessed October 8, 2024).
to identify data gaps and establish funding priorities, such as improving the efficiency, timeliness, and quality of data capture and access; enhancing linkages to current data; and filling gaps in current data collection.
Cancer Surveillance and Registries in Europe
Europe has 180 population-based cancer registries across 41 countries, covering about two thirds of the population of Europe (Arndt and Siesling, 2024), said Volker Arndt, director of the Baden-Württemberg Cancer Registry and head of the Unit of Cancer Survivorship at the German Cancer Research Center or Deutsches Krebsforschungszentrum (DKFZ).43 “Registration practices differ, and harmonization is a key task of the European Network of Cancer Registries,”44 he said, adding that the European Commission has recently committed €13 million to support quality improvement of European cancer registry data. Arndt shared his perspective on cancer surveillance and registries based on his experience with cancer registration in Germany.
Arndt said that prior to the Federal Cancer Registry Act in 1995,45 Germany had only a few cancer registries (Robert Koch Institute, 2016). By 2009, all states had registries (Robert Koch Institute, 2019). Additional legislation followed in 2009, addressing the collection and sharing of harmonized data at the federal state-level;46 in 2013, enabling quality assurance through registries;47 in 2021, addressing access to cancer registry data;48 in 2022 a call was launched to leverage AI to enhance the use of registry data;49 and, in 2024, addressing linkage of cancer registry and other data for research use.50 Arndt said that Germany now has 15 federal state-level cancer registries and one nationwide childhood cancer registry. “All data are pooled at the national level,” Arndt added (Katalinic et al., 2023).
___________________
43 See https://www.dkfz.de/en/krebsregister/index.html (accessed October 16, 2024).
44 See https://encr.eu (accessed December 16, 2024).
45 The Federal Cancer Registry Act was passed in Germany parliament in November 1994 and was in effect from 1994 to 1999. See http://www.bgbl.de/xaver/bgbl/start.xav?startbk=Bundesanzeiger_BGBl&jumpTo=bgbl194s3351.pdf (accessed November 23, 2024).
46 See https://www.gesetze-im-internet.de/bkrg (accessed November 23, 2024).
47 See http://www.bgbl.de/xaver/bgbl/start.xav?startbk=Bundesanzeiger_BGBl&jumpTo=bgbl113s0617.pdf (accessed November 23, 2024).
48 See http://www.bgbl.de/xaver/bgbl/start.xav?startbk=Bundesanzeiger_BGBl&jumpTo=bgbl121s3890.pdf (accessed November 23, 2024).
49 See https://www.bundesgesundheitsministerium.de/ministerium/ressortforschung/handlungsfelder/forschungsschwerpunkte/krebsregisterdaten.html (accessed November 23, 2024).
50 See https://www.gesetze-im-internet.de/gdng (accessed November 5, 2024).
Arndt highlighted some of the key characteristics of population-based cancer registration in Germany, including a comprehensive data catalog of more than 130 items spanning tumor information, primary and subsequent treatment, recurrence and progression, genetics, and other information.51 He said that reporting is mandatory for clinicians involved in the treatment of the patient’s cancer and patient consent is only required for follow-up contact for future research purposes, such as recruitment of survivors to obtain further information. Additionally, data can be exchanged between states, allowing for longitudinal tracking of patients who move or receive care in a different state. Arndt said that clinicians receive a financial incentive for reporting (Katalinic et al., 2023). He added that an approach to capturing patient-reported outcomes is in development.
Arndt also discussed several examples of the applications of population-based cancer registry data, such as tracking quality indicators of cancer care (e.g., for a particular type of cancer or by type of hospital); understanding the patient experience (e.g., travel distance to treatment and willingness to travel further for a certified cancer center); and studying real-world outcomes (e.g., recurrence rates for a particular cancer relative to treatment and other factors) (Holleczek et al., 2023; Klora et al., 2024; Schulz et al., 2024).
Arndt noted that challenges with cancer registration still persist including data quality (e.g., completeness and timeliness); increased workload for clinicians and registry staff; the need for data validation and harmonization; and costs. The initiative in Germany to leverage AI to enhance the use of registry data and the recently introduced legislation on linking cancer registry data with health insurance data52 could help overcome some of these challenges, he concluded.
DATA COLLECTION METHODOLOGIES AND TECHNOLOGICAL ADVANCES IN CANCER SURVEILLANCE
Peter Paul Yu, physician-in-chief of the Hartford HealthCare Cancer Institute and Clinical Member at the Memorial Sloan Kettering Cancer Center (MSK), said that data standards including data dictionaries describing elements of importance for cancer reporting have been developed over decades by multiple organizations such as NPCR, SEER, and NCDB. He noted, however, that these can be specific to each organization’s use cases and do not necessarily align with one another. New data element lists continue to be developed, including
___________________
51 See https://www.basisdatensatz.de/basisdatensatz (accessed October 16, 2024).
52 See https://www.recht.bund.de/bgbl/1/2024/102/VO.html (accessed October 16, 2024).
USCDI+53 for cancer, a domain-specific list to accompany the U.S. Core Data for Interoperability (USCDI) dataset54 maintained by the Office of the National Coordinator for Health IT (ONC). Attention is also turning to the need for data elements to describe information derived from omics-based tests.55 The challenges, Yu said, include data capture in both human and machine-readable formats (e.g., structured data entry in EHRs and synoptic pathology reports), harmonization of data dictionaries, and adoption of transmission standards (e.g., state cancer registry capabilities and bidirectional data sharing). There are emerging methods, such as common data models, but he noted that new approaches can be complex, and they could create additional burdens for data entry. There are potential roles for new technologies, such as generative AI, and knowledge graph56 extensions, which can capture relevant data from existing sources (e.g., social determinants of health information from census data). The ultimate goal of capturing data is to inform a learning health care system (see Figure 3) and improve care quality and value, which Yu said involves applying data science and machine learning to high quality digital data to answer key questions.
Many panelists representing a range of organizations involved in data collection and curation for cancer surveillance shared their perspectives on current approaches and emerging tools for automation and analytics.
Curating Real-World Electronic Health Record Data with the Flatiron Health Database
Emily Castellanos, senior medical director at Flatiron Health, said that Flatiron Health curates real-world data from more than 3 million patient records across a network of oncology practices (Castellanos et al., 2024). About three quarters of the network is community oncology practices that
___________________
53 See https://www.healthit.gov/topic/interoperability/uscdi-plus (accessed October 14, 2024).
54 See https://www.healthit.gov/isp/united-states-core-data-interoperability-uscdi (accessed October 16, 2024).
55 Omics refers to any of several areas of biological study defined by investigating the entire complement of a specific type of biomolecule or all of a molecular process within an organism, such as proteomics, metabolomics, genomics, and transcriptomics. See https://www.britannica.com/science/omics (accessed October 14, 2024).
56 Knowledge graphs are models used to organize interlinked concepts to connect data and context, thereby empowering analytics, integration, and human–system interfaces. See https://www.turing.ac.uk/research/interest-groups/knowledge-graphs (accessed October 16, 2024).
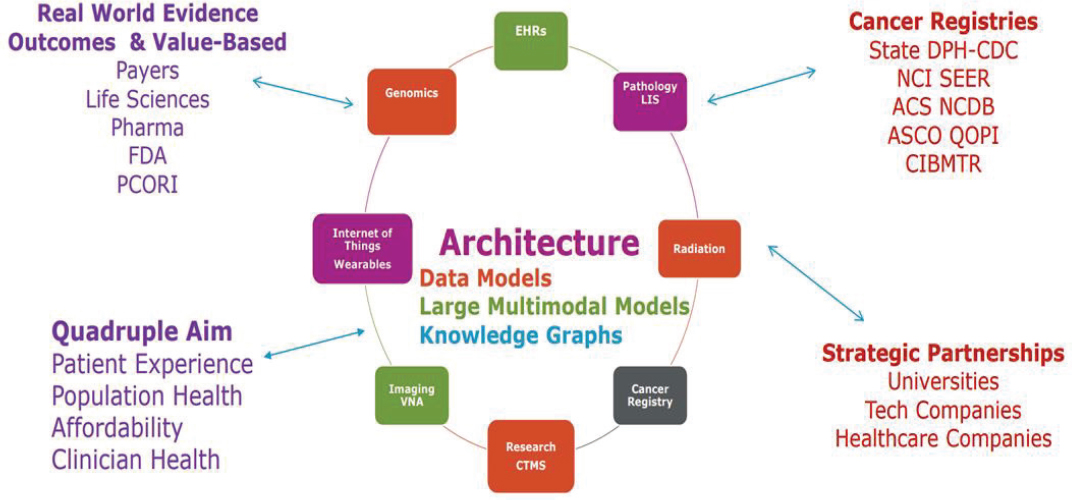
NOTES: ACS = American College of Surgeons; ASCO = American Society of Clinical Oncology; CIBMTR = Center for International Blood and Marrow Transplant Research; CTMS = Clinical Trial Management System; DPH-CDC = Division of Population Health-Centers for Disease Control and Prevention; FDA = U.S. Food and Drug Administration; LIS = laboratory information system; NCDB = National Cancer Database; NCI = National Cancer Institute; PCORI = Patient-Centered Outcomes Research Institute; QOPI = Quality Oncology Practice Initiative; SEER = Surveillance, Epidemiology, and End Results; VNA = vendor neutral archive.
SOURCE: Yu presentation, July 29, 2024.
use Flatiron’s EHR product, OncoEMR,57 and about one quarter is academic medical centers for which Flatiron Health integrates into the local EHR system (Guadamuz et al., 2023).
Real-world data can be found in both structured and unstructured formats in the EHR, Castellanos said, adding that mapping and harmonization of structured data are necessary because formats can vary by site. Much of the real-world data in the EHR is in unstructured documentation (e.g., clinician notes, pathology reports, and radiology reports), which is converted into structured variables through human abstraction, machine learning, and/or natural language processing. Flatiron Health also links to patient data from outside sources, such as genomic data, claims data, socioeconomic status information, Social Security death index data,58 and obituary information, she said (Castellanos et al., 2024).
Castellanos shared an example of the breadth of critical, complex clinical information that can be captured from unstructured data, such as those in imaging or biomarker reports. However, systematic abstraction of unstructured data is resource intensive and requires staff training and standardization of policies and procedures to ensure continuity across organizations and time. Quality assurance is also necessary, including assessing abstractor agreement, assessing and adjudicating discrepant or implausible data, and validating form entries to reduce human error, she said (Castellanos et al., 2024).
Castellanos said that Flatiron is developing machine learning and AI approaches to augment human abstracting. She described one approach that trains the software to use regular expressions59 to find certain phrases to determine how something might be documented in a chart. She said that teams of clinical experts work with the software engineers to inform the training of the machine learning models with selected phrases and labeled abstracted data. She added that “the model learns which phrases indicate metastatic diagnoses based on training examples, from which it will generate predictions.”
Castellanos discussed several challenges for advancing abstraction of real-world data from EHRs. The first is how to ensure data quality for machine learning and AI approaches. Another is determining “the right balance between humans and technology,” and she said that it is unlikely that machine learning extraction will be done without any human involvement. Finally, she raised the question of how to add and quickly scale new variables. “Will there be new techniques which will allow us to scale without having to abstract thousands of patients?” Castellanos queried.
___________________
57 See https://flatiron.com/oncology/oncology-ehr (accessed October 16, 2024).
58 See https://www.ssa.gov/dataexchange/request_dmf.html (accessed October 16, 2024).
59 Regular expressions are specific syntax used to search for terms. See https://www.csfieldguide.org.nz/en/chapters/formal-languages/regular-expressions (accessed December 16, 2024).
The National Cancer Database Rapid Cancer Reporting System for Real-Time Reporting
Palis said that the NCDB invested in the RCRS, a real-time data collection infrastructure, in direct response to feedback from CoC-accredited cancer programs. He said that the programs expressed their need for real-time data to support expedited reporting of key quality of care metrics for benchmarking hospital performance.
Per CoC standards, local registries at CoC-accredited programs now report all newly diagnosed cancer cases to the RCRS at least monthly,60 Palis said. It is also expected that staging, first course treatment,61 and follow-up data will be reported when available, which he said is “an incremental move towards concurrent abstraction.” Palis noted that moving from submission of complete record layout data to incremental reporting with concurrent abstraction is a “fundamental shift in the way that registries operate” and that the American College of Surgeons is developing relevant standards and technology enhancements. He added that the benefits of continued enhancements to the NCDB real-time reporting platform will only be realized if registries have automation tools to reduce their workload.
To improve the accuracy of case submissions, interactive data quality reports provide programs with information on the completeness of reported data for required items. Programs can monitor their quality measure performance “in real-time from data updated daily,” Palis said, and see their rolling performance rate for the prior 12 months. Hospitals can also view their performance benchmarked against others (ACS, 2022). Another enhancement, which will be launched in 2025, is the capability for CoC-accredited programs to create interactive benchmark reports based on real-time data and submit these to NCDB, Palis concluded.
Reconceptualizing Electronic Health Record Data Entry: The Penn Smart Form Documentation Tool
Shulman said that critical patient data are largely in free text, and they are inconsistently reported in EHRs. It is important to recognize that automated data extraction technologies can only extract what is in the EHR, he added. More complete data can also help elucidate why a patient’s clinician
___________________
60 See https://www.facs.org/quality-programs/cancer-programs/commission-on-cancer/standards-and-resources/2020/implementation (accessed October 23, 2024).
61 First course treatment includes “all types of treatment documented in the treatment plan and given to the patient prior to progression of disease or recurrence of disease.” See https://training.seer.cancer.gov/treatment/first-course (accessed December 17, 2024).
has made particular treatment choices. To enhance the content in EHRs, the University of Pennsylvania has developed and implemented an EHR-based documentation tool that prompts the entry of key data in a structured format. Shulman said that “the smart form,” together with cancer staging and routinely collected patient-reported outcomes and other structured data in the EHR (drugs, doses, lab results, etc.) will give a more comprehensive picture of persons with cancer across their care continuum. He emphasized that the form facilitates documentation, does not add work, and need not be reiterated in a free text entry.
Shulman also described a recent study that looked at the impact of payment policies on how clinical information is documented. Clinicians focus on information required for reimbursement, he said, and the result is “poor-quality, low-value documentation that is not structured or computable and contributes to clinician burnout” (Gabriel et al., 2023). The authors proposed a payment approach to reimburse for documenting key data in a structured format.
Lessons From the COVID-19 and Cancer Consortium
Jeremy Warner, founding director of the Center for Clinical Cancer Informatics and Data Science at Brown University, said informatics requires people, process, and technology. During the COVID-19 pandemic, the ability to gather timely data on persons with cancer who were diagnosed with COVID-19 was lacking. The people and technology were there, Warner said, but the processes were missing. The COVID-19 and Cancer Consortium (CCC19)62 was formed to fill the gap. The consortium now includes 123 institutions and more than 600 investigators.
Warner provided information on some essential elements that needed to be rapidly assembled to launch CCC19, from overall governance of the consortium to committee structures, the data model, terminology, quality metrics, analytics, and outreach and patient engagement. Warner noted that processes were copied from other organizations when possible, and persons with cancer who were experiencing COVID-19 were engaged to inform the development of the database.
Echoing a number of other speakers, Warner said that critical elements needed to populate the CCC19 registry, such as cancer treatment regimens, treatment toxicity, cancer status, and performance status, were not found in structured EHR data and often not in unstructured data either (Kim et al., 2019).
___________________
62 See https://ccc19.org (accessed October 16, 2024).
In summarizing the lessons learned from CCC19, Warner said that “goodwill was critical but can be fleeting” and some type of reward is necessary to sustain engagement. Studies found that the main factors that drove outcomes were those for which data were often missing or represented only in free text, such as cancer status, Eastern Cooperative Oncology Group (ECOG) performance status,63 institutional factors (e.g., differences in surgical outcomes), and COVID-19 vaccination status (Grivas et al., 2021; Hawley et al., 2022; Kuderer et al., 2020; Schmidt et al., 2022). He said data entries of “unknown” were allowed and missingness was tolerated, but they were taken into account in quality metrics (CCC19, 2020).
The Centers for Disease Control and Prevention Electronic Pathology Implementation Project
Sandy Jones, public health advisor in the cancer surveillance branch at CDC, said that the agency has made significant progress with implementing real-time electronic pathology reporting from laboratories to central cancer registries. However, the lack of structured pathology data presents a persistent challenge and can hamper the identification of reports that should be submitted to registries.
CDC developed the Electronic Mapping, Reporting, and Coding (eMaRC) Plus64 software in 2005 to facilitate the receipt and processing of narrative and synoptic pathology reports by central registries. Jones also described how CDC has used the eMaRC Plus rule-based language model to develop eMaRC Lite,65 a new tool to process narrative pathology reports that reduces the amount of manual review needed. eMaRC Lite determines reportability and auto-codes four required data elements: histology, behavior, primary site, and laterality. More than 30 hospital-based and central cancer registries are currently working with eMaRC Lite. Initial results have shown that a false negative rate of less than 1 percent can be achieved, but, Jones said, “the model will need to be monitored for accuracy and retrained on a regular basis.”
___________________
63 The ECOG Performance Status Scale, developed by the Eastern Cooperative Oncology Group (ECOG), now known as the ECOG-ACRIN Cancer Research Group, was published in 1982. This scale assesses a patient’s level of functioning, with and without restriction, using a variety of factors, including the ability to care for oneself, daily activity, and physical ability (e.g., walking, working). This scale provides a grade from 0 (fully active, no restrictions) to 5 (deceased). See https://ecog-acrin.org/resources/ecog-performance-status (accessed October 23, 2024).
64 See https://www.cdc.gov/national-program-cancer-registries/registry-plus/emarc-plus.html (accessed October 16, 2024).
65 See https://info.cap.org/future-of-cancer-data-summit-insights/downloads/NPCR-ePath-FactSheet.pdf (accessed October 30, 2024).
Jones said that CDC is working to improve the timeliness of cancer reporting. She elaborated on CDC’s partnership with APHL to use its AIMS platform to enable daily reporting to registries by pathology laboratories. The effectiveness of this infrastructure for daily cancer pathology reporting has been demonstrated by nearly 200 laboratories across the United States.66 She added that CDC is developing a national cloud-based platform to enable secure, real-time reporting of cancer cases to central registries. The platform will also offer shared tools and services for data processing and analysis. For example, as part of its efforts to improve timely reporting of childhood cancers, CDC launched the NPCR National Oncology rapid Ascertainment Hub (NPCR NOAH).67 This system supports laboratories in reporting pathology data in standard HL7 format. Faster reporting through CDC’s cloud-based platform supports timely assessment of interventions, resource allocation, clinical trial recruitment, and identification of research priorities. Jones also discussed the Trusted Exchange Framework and Common Agreement (TEFCA),68 an infrastructure that facilitates the timely exchange of high-quality, standardized data among Quality Health Information Networks (QHINs).69 She emphasized the importance of implementing data standardization for successful data sharing among health care systems “so that systems can automatically populate the appropriate fields in the EHR with data received from other health care systems.”
CodeX Minimal Common Oncology Data Elements
Su Chen, clinical science principal at MITRE and co-chair of the CodeX HL7 Fast Healthcare Interoperability Resources (FHIR), Accelerator discussed leveraging open common data models to advance cancer data interoperability at CodeX.70 Chen explained that CodeX is an HL7 FHIR accelerator, which is a member-driven community focused on advancing clinical specialty data standards, such as minimal Common Oncology Data Elements (mCODE™),
___________________
66 See https://www.cdc.gov/national-program-cancer-registries/data-modernization/advancing-electronic-reporting.html (accessed October 23, 2024).
67 See https://www.cdc.gov/national-program-cancer-registries/about/pediatric-young-adult-cancer.html (accessed October 16, 2024).
68 See https://www.healthit.gov/topic/interoperability/policy/trusted-exchange-framework-and-common-agreement-tefca (accessed October 19, 2024).
69 A Quality Health Information Network (QHIN) is a network that directly connects participating organizations to achieve interoperability. The qualification process includes completing an application, onboarding, and designation. See https://www.healthit.gov/topic/interoperability/policy/trusted-exchange-framework-and-common-agreement-tefca (accessed October 19, 2024).
70 See https://www.hl7.org/codex (accessed October 23, 2024).
to improve patient experience and outcomes in real care settings. A broad range of CodeX use cases are underway, including a pilot focused on cancer registry reporting.
mCODE71 is a core set of common cancer data based on the FHIR common health data exchange standard. Chen explained that mCODE is an open data model that is standardized and affords API capabilities, which can help to implement digital health solutions at scale. She said that standards are only valuable if they are used widely, effectively, and in actual health care settings. Chen noted significant uptake by vendors, with about half of U.S. patient records in vendor systems that use mCODE. A recent study at the Mayo Clinic found that mCODE used in the context of routine patient care increased data capture with minimal to no clinician burden, she continued (Tevaarwerk et al., 2024). In addition, mCODE is now being used for data capture beyond academic hospitals in community cancer clinics.
Now is the time to reshape the cancer data landscape, Chen said, emphasizing that industry, community, and federal agency efforts are aligning, with momentum building from federal agency cancer initiatives, such as the Cancer Moonshot.72
The OneFlorida+ Clinical Data Trust Model for Facilitating Research
Elizabeth Shenkman, professor and chair of the Department of Health Outcomes and Biomedical Informatics at the University of Florida, discussed the OneFlorida+73 Clinical Data Trust74 as a model of a central data repository to facilitate research. OneFlorida+ partners with academic, regional, and statewide health systems across Florida and select partners in Alabama, Arkansas, California, Georgia, and Minnesota and is a PCORnet75 network partner. At the core of OneFlorida+ is the central data trust, which contains EHR data for over 26 million patients spanning 2012 to present, she said.76
Data in the OneFlorida+ Data Trust are derived from EHRs and other sources (see Figure 4) and stored in the formats of the Observational Medical Outcomes Partnership (OMOP) Common Data Model77 and PCORnet
___________________
71 See https://hl7.org/fhir/us/mcode (accessed October 23, 2024).
72 See https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative (accessed November 1, 2024).
73 See https://onefloridaconsortium.org/about (accessed October 17, 2024).
74 See https://oneflorida.sites.medinfo.ufl.edu/data-2 (accessed October 17, 2024).
75 See https://pcornet.org (accessed November 2, 2024).
76 See https://oneflorida.sites.medinfo.ufl.edu/data-2 (accessed December 4, 2024).
77 See https://www.ohdsi.org/data-standardization (accessed October 17, 2024).

NOTES: ETL = extract, transform, and load; ID = identification; IRB = institutional review board.
SOURCES: Shenkman presentation, July 29, 2024; OneFlorida+ Clinical Research Network, 2024.
Common Data Model,78 Shenkman said. Other elements of the model include privacy-preserving record linkage; data use agreements with partners; and the ability to reidentify patients, with institutional review board (IRB)79 approval, for potential participation in clinical studies.
Shenkman said that very important clinical information, including information on symptoms, adverse events, social determinants of health, and family history, are locked in EHR text notes. To address this, OneFlorida+ developed GatorTron, a large clinical language model for transforming unstructured clinical data for entry into the Data Trust (Yang et al., 2022a) and GatorTronGPT (generative pre-trained transformer), a generative clinical large language model (Peng et al., 2023). Computing capacity and infrastructure were provided through a partnership with NVIDIA.80
Shenkman shared two recent studies that used data in the OneFlorida+ Data Trust. One study used the data to identify individuals at greatest risk for lung cancer to better target screening efforts and monitor the benefits and harms of lung cancer screening (Yang et al., 2022b). The other linked the data to EHR data and Florida SHOTS81 data to identify clinician practices with low human papillomavirus (HPV) vaccination rates and evaluate targeted interventions for increasing vaccination.82
Core Clinical Data Elements for Interoperable EHRs in Cancer Surveillance
Peter Stetson, chief health informatics officer at MSK, said, “We want to predict cancer onset, tumor evolution, treatment response, [and] toxicity burden so we can improve care outcomes and value.” However, the ability to leverage AI-based methods in oncology is limited by the ability to obtain high-quality, shareable data.
Stetson described how MSK is approaching this problem using a real-world data model, the Core Clinical Data Elements (CCDE). CCDE is an OMOP-based model drawing on existing models, including NAACCR,
___________________
78 See https://pcornet.org/data/common-data-model (accessed October 17, 2024).
79 IRBs are committees that oversee research involving human subjects to protect human rights and welfare. See https://www.fda.gov/about-fda/cder-offices-and-divisions/institutional-review-boards-irbs-and-protection-human-subjects-clinical-trials (accessed October 17, 2024).
80 See https://blogs.nvidia.com/blog/gatortrongpt (accessed October 17, 2024).
81 See https://flshotsusers.com (accessed October 17, 2024).
82 See https://clinicaltrials.gov/study/NCT05006833?term=Text (accessed November 26, 2024).
mCODE, and others. He said that MSK is deep phenotyping83 a cohort of patients for whom deep tumor genotyping data are available from the MSK-Integrated Mutation Profiling of Actionable Cancer Targets (IMPACT®) genotype panel.84 He explained that by using CCDE, MSK has treatment and outcomes data for a cohort of 24,000 deeply phenotyped patients. These data are highly curated and structured, have greater than 95 percent accuracy, and are used by MSK as a reference standard for AI model development and validation of vendor models, Stetson added.
Stetson said that the benefits of the CCDE real-world data model are that it is “relieving the data quality constraint for AI,” offers a “proving ground” for AI model development, and enables data sharing outside of MSK. He added that AI-enabled curation also increases data density more quickly.
Stetson offered his perspective on a road map for moving forward, including creating an automated cancer registry on a national scale; “federated tuning of AI-enabled curation,” establishing academic–industry partnerships to facilitate a “computable phenotype ecosystem,” and enabling “cross-institutional computable journeys” to be able to predict treatment response based on full patient history across time and place of care.
Emerging Directions in Multimodal Health Data Analytics
Peter Clardy, lead of the Clinical Enterprise Team at Google Health, discussed opportunities to integrate AI tools to manage multimodal patient data. Traditional programming systems involve structured inputs, algorithms, and outputs that are highly deterministic, he said. The original AI and machine learning tools involved supervised learning, which Clardy said “would allow datasets that are highly curated with a ground source of truth to perform certain kinds of task-specific data transformations.” Emerging generative language models, such as GPT,85 learn from consuming huge volumes of data and then use an inference process for tasks, such as answering questions or data abstraction.
Clardy described three waves of AI, beginning in the 1980s with limited capabilities and evolving in the 2010s to neural networks that could successfully complete narrow tasks but could be fragile, required expensive training data, were poor at multimodal and sequential data, and could be difficult to interpret (Sharma et al., 2020). Now, in the third wave, the cost of training is lower,
___________________
83 In precision medicine, deep phenotyping is an emerging process that gathers more specific, individualized details on disease manifestations and presentations to better understand potential connections between genetic variations and disease subtypes (Delude, 2015).
84 See https://www.mskcc.org/msk-impact (accessed October 18, 2024).
85 See https://www.ibm.com/think/topics/gpt (accessed October 24, 2024).
and generative AI is more capable of handling multimodal and sequential data, resulting in “rich, expressive outputs,” he said (Howell et al., 2024).
The use of AI in health care was initially focused on what Clardy described as “mid-generation supervised learning tasks,” such as lung or breast cancer detection, pathology predictors, and genomics (Howell et al., 2024). Today, the focus is on multimodal models that can integrate data from a range of sources and formats, learn, and create a range of outputs for different applications (Acosta et al., 2022). As mentioned, a challenge for AI is that “longitudinal comprehensive patient records are multimodal, heterogeneous, and laden with unstructured data,” Clardy said. Other challenges are that health-related questions are “complex and nuanced,” and the more preprocessing of the questions and the data that are done, the more rigid and costly the systems become. And, while tools such as FHIR have increased data interoperability, capturing information from unstructured data is a persistent challenge.
Clardy noted many opportunities to integrate AI tools into health care (e.g., knowledge graphs, optical character recognition tools, and multimodal patient data management), and each will have advantages and disadvantages. Current systems are still relatively deterministic, but he said there is an increasing number of human-in-the-loop hybrid AI tool pilots. Clardy added that although it is still in the proof-of-concept stage, a generative future is emerging, where large models can extract useful individual- and population-level data from large volumes of patient records. A consideration, however, is that clarity regarding how these systems work decreases as the use of generative tools increases, Clardy concluded.
Getting Data into the Record
Several session participants commented on a range of topics related to populating the EHR with the data needed by cancer registries.
Data Elements
Shulman reiterated that essential data elements include cancer recurrence and factors that provide information about why a patient’s clinician has made particular care decisions. The challenge, he said, is balancing “all the things we might want to know with the things that we absolutely have to know.” Chen highlighted the need to consider what other data elements might be needed for clinical actionability. Another area where data elements are lacking across data models is survivorship. She raised a concern that creating “different siloed versions of these data elements” could exacerbate interoperability barriers. Chen called for cross-sector collaboration to establish a cohesive platform that does not add burden, and Yu noted the need for public–private collaboration
to develop a shared vision. Warner said that cancer is many different diseases and suggested there is a need to consider when highly detailed data are needed versus when a subset is sufficient. He added that, whether machine or human, effort is finite, and one cannot have all the elements on every cancer.
Large Language Models
Gabriel observed that large language models work well with natural language and perhaps less so with medical language and lingo. He mentioned ambient listening as an emerging technology that might be used to extract information from clinical conversations (e.g., patient–clinician conversations and tumor board meetings) and provide prose for AI models to transform into structured data. Clardy said the patient record is often a poor reflection of a patient’s situation due to problems such as data missingness, copy-and-paste text, coding limitations, and lack of record interoperability (Turbow et al., 2021; Wells et al., 2013). “The language of health is multimodal and expanding, and an incremental approach with a human in the loop is needed,” Clardy added.
Incentives
Shulman said that the quality of clinic notes varies widely and reiterated that the data need to be available to be extracted. He noted that inadequate data entry is not malicious but rather the result of the numerous priorities competing for the clinician’s time. Current payment systems do not reward quality data entry, and he said incentives are needed to ensure clinicians document critical data elements at every patient encounter. For example, incremental reimbursement for a pathology report could be contingent on the report being in a synoptic format with structured data. Shulman also highlighted the need to eliminate unnecessary data elements in the EHR and include what matters most.
Warner pointed out the disconnect between what the clinical community needs to care for its individual patients and what the registry community needs at the population level for public health. He added that clinicians do not generally receive any training on cancer registries, and he suggested education is needed so that clinicians understand why registries are important for their work.
Yu said continuing to add more data elements contributes to clinician workload and burnout. Although smart forms can facilitate data entry, he said, many clinicians are resistant to clicking through a form and still prefer narrative, even as a redundant task that adds time. Jones noted the software tools that enable the clinician to speak their responses to the form questions. She said one issue is that clinicians are overwhelmed and should not spend their time searching for codes.
Data Linkages
Castellanos said there are benefits to linkages, but generalizability and representativeness can be concerns. She explained that the linked source must contain the data desired, and elements such as recurrence data are difficult to obtain unless manually curated. She also noted the importance of being able to accurately match a patient in both sources. Another challenge, Castellanos said, is that patients will be missing from linkages. For example, claims data might not include all insurance plans covering a patient and would not include patients without insurance. Also, not everyone treated for cancer in the United States has a Social Security number or other identifiers that supports data linkage. Combining linkages in one dataset (e.g., genomics, claims, EHR, and vital status data) can result in a narrower population than originally intended, she said. The result is that the data for those patients in the linkage might have a high level of completeness, but not all relevant patients will be included.
Closing the Gaps
Hanson said there is a need to identify what infrastructures are still lacking to be able to achieve the vision for national-level cancer surveillance, encompassing all populations, in near real time. Robert Winn, director of the Virginia Commonwealth University Massey Cancer Center, said that closing gaps in cancer surveillance data requires both “high tech and high touch.” He suggested that one approach to capturing data for populations missing from registries is to reimagine the workforce to increase data collection and dissemination in rural and underserved areas, create jobs, and use a high-touch approach to gather data where needed.
Making the Business Case for Investment in Data Collection Infrastructure
Participants also discussed the importance of the business case for organizational investment in infrastructure to support data entry and extraction to expand the registry population. Chen said that one business driver for users adopting common data models is the significant time, expense, and expertise needed to develop their own data models for a complex disease, such as cancer. She emphasized the importance of considering the purpose of design when investing resources to implement an approach. For example, she said, FHIR and OMOP were designed for different purposes: real-time health care information exchange and retrospective research, respectively.
Clardy agreed with the need to understand the distinctions among data models and to build for purpose. From a business case perspective, he agreed
with an “incrementalist approach” of applying technology to improve operational efficiencies, such as automating tasks to reduce the drain on staff resources. Clardy said that improved long-term outcomes, such as lives saved, are very difficult to demonstrate, but hours saved is generally a metric that all parties can support. He also cautioned that “there is a tendency to use generative AI to solve for everything,” which he said has “the potential for very non-deterministic outcomes and is also computationally extremely expensive and time-consuming.” Clardy recommended “working up the level of complexity and risk … and applying deterministic or machine learning systems where you can, and then only leveraging these more generative models when nothing else will do” and making sure to include “human-in-the-loop and process supervision.”
THE INFRASTRUCTURE AND WORKFORCE SUPPORTING CANCER SURVEILLANCE
Several speakers discussed issues related to the evolving infrastructure for cancer surveillance and the shifting role of the workforce, with a focus on ensuring the capture of high-quality clinical data and reducing abstraction time.
Infrastructure
College of American Pathologists Cancer Protocols: Harnessing Structured Data to Optimize Cancer Surveillance
The vision for the College of American Pathologists (CAP) Cancer Protocols is to “improve patient outcomes through structured cancer pathology reporting solutions,” said Samantha Spencer, senior director of Standards and Guidelines at CAP. Protocols are based on current standards and include the core elements required by accreditation organizations (e.g., the American College of Surgeons, CoC, and CAP Laboratory Accreditation Program).86 There are 101 CAP Cancer Protocols,87 and templates for reporting by pathologists are available in MS Word and PDF formats and as electronic forms integrated into laboratory information systems (LIS) that can generate synoptic reports.88
To create and edit the electronic Cancer Protocols, CAP developed a robust, web-based content generation tool based on the structured data
___________________
86 See https://www.cap.org/laboratory-improvement/accreditation/laboratory-accreditation-program (accessed October 19, 2024).
87 See https://www.cap.org/protocols-and-guidelines/cancer-reporting-tools/cancer-protocols (accessed October 19, 2024).
88 See https://www.cap.org/protocols-and-guidelines/cancer-reporting-tools/cancer-protocol-templates (accessed October 8, 2024).
capture object model,89 Spencer explained. This approach facilitates capture of structured data within the medical record; mapping of data elements to standardized terminologies, including SNOMED CT90 and ICD-O; and transmission of structured, standardized data. Spencer noted that vendor implementation of the electronic Cancer Protocols is a key to success in clinical practice. To ensure successful vendor implementation, CAP works collaboratively with vendors, promotes bidirectional communication and feedback, and conducts implementation validation sessions to ensure fit into the workflow. The next step, which is still in development, is facilitating data transmission to downstream users, including registries (Torous et al., 2021).
Spencer emphasized that multidisciplinary collaboration is essential for realizing CAP’s vision for structured pathology reporting, and CAP is engaging with public health partners and standards-developing organizations on a range of topics. CAP is also engaged in commenting on federal health IT initiatives, such as USCDI, USCDI+, and HTI-2.91
A challenge, as discussed throughout the workshop, is that data in the clinical record are frequently not structured or standardized, Spencer said, and “human interpretation and translation is still needed.” There are also challenges with standardized data coding, and she said version control also needs to be addressed.
The common vision, Spencer summarized, is to improve patient care through interoperable cancer data reporting and downstream data use (Torous et al., 2021). Education and training are critical elements of success, and she noted the need to provide training about registries early in medical education. She also referred participants to the CAP Cancer Data Champions webinar series.92 Focus on implementation, work with vendors and end users to understand their needs and ensure functionality in clinical workflows, and support adoption at a national level through coordination, collaboration, and change management, she concluded.
Use of EHRs and Health Information Exchanges to Streamline Registry Processes
Mary Charlton, professor of epidemiology and director of the Iowa Cancer Registry93 at the University of Iowa College of Public Health, dis-
___________________
89 See https://www.healthit.gov/topic/scientific-initiatives/pcor/research-evaluation/structured-data-capture-sdc (accessed October 19, 2024).
90 See https://www.snomed.org/what-is-snomed-ct (accessed October 24, 2024).
91 See https://www.healthit.gov/sites/default/files/page/2024-07/HTI-2_ProposedRule_Overview_Factsheet_508.pdf (accessed October 24, 2024).
92 See https://www.cap.org/calendar/webinars/cancer-data-champions (accessed October 8, 2024).
93 See https://shri.public-health.uiowa.edu (accessed October 24, 2024).
cussed the role of EHRs and health information exchanges (HIEs) in cancer registration. HIEs facilitate the electronic transmission of patient medical information among health care-related professionals and facilities,94 and she said that most states have one or more (ONC, 2019).
It is essential to collect data from all patients, not just those receiving care at academic medical centers, Charlton said. In Iowa, many patients in rural areas receive cancer care at one of 81 critical access hospitals, and not all of these hospitals have an oncologist (Schroeder et al., 2022). She said that HIEs can be a mechanism for registries to access EHR data for their entire catchment area, rather than collecting data from each hospital. The use of this approach is “highly variable across cancer registries and generally in early stages,” she said, and most registries are doing manual abstraction. Although technology has a role in helping registries collect an increasing number of data elements in the face of declining resources, Charlton said that technology cannot fully replace people, and human relationships can be essential to gathering data from rural hospitals.
Charlton described several lessons learned from linking the Iowa Cancer Registry to EHR data in CancerLinQ.95 As mentioned by several other speakers, successful linkages with EHRs require good identifiers (e.g., Social Security number, full name, birth date, sex, race, and address), and this can be very challenging in states with strict policies regarding protected health information, she said. Another challenge for registries is linking EHR data for persons with multiple cancers. As an example, she said, if a patient had one tumor in each breast, data were insufficient to “accurately link EHR data to the correct tumor.” Linkage of the registry to EHR data in CancerLinQ was beneficial for improving the capture of treatment data, she said, particularly hormone and immunotherapy data (Charlton et al., 2022). There are also efforts to automate data extraction from EHRs, and she mentioned ExtractEHR96 as an example, which is “an R97 software package to automate laboratory adverse event ascertainment and grading” in pediatric cancer (Miller et al., 2022). She described the progress as promising and said that “in the future, these activities,
___________________
94 See https://www.healthit.gov/topic/health-it-and-health-information-exchange-basics/what-hie (accessed October 24, 2024).
95 For information on CancerLinQ, see https://www.cancerlinq.org/. In December of 2023, CancerLinQ ownership was transferred from the American Society of Clinical Oncology to ConcertAI. See https://connection.asco.org/magazine/features/building-cancerlinq-future-next-step-ambitious-asco-project (accessed October 19, 2024).
96 See https://www.cancer.gov/research/areas/childhood/childhood-cancer-data-initiative/progress/projects (accessed October 24, 2024).
97 R is a programming language for statistical computing and data visualization. See https://www.r-project.org/ (accessed November 5, 2024).
coupled with more advanced interoperability standards, could allow for more efficient flow and use of EHR data through [HIEs] to registries.”
There are also opportunities to leverage HIEs to streamline registry processes beyond EHR data, Charlton said. One example is for case finding, which she said, “can be a tedious and resource intensive process for hospitals, as well as for registries,” especially in rural states. Charlton said HL7 notifications to admit, discharge, and transfer (ADT)98 likely contain variables useful for linkage and could be used for case finding, as well as for auditing the completeness of hospital case ascertainment. She noted that the Iowa Cancer Registry has partnered with CyncHealth (the HIE in Iowa)99 to use ADTs for case finding because nearly all of Iowa’s hospitals share data with this HIE. As another example, she said, the Maryland Cancer Registry is partnering with the state HIE, the Chesapeake Regional Information System for Our Patients,100 to find information on persons who are found to have cancer only at the time of death. Charlton pointed out that patients with insufficient registry data are generally excluded from research, and HIEs can be used to find the needed information.
Jackie Gerhart, chief medical officer at Epic, discussed how AI in EHRs can empower the cancer care workforce. For example, rule-based logic provides clinical decision support, such as flagging cancer screenings the patient is due for. At the other end of the spectrum is generative AI, and she emphasized that such tools to support the workforce should be fit for purpose (i.e., using AI only when needed).
Castellanos also discussed EHR technologies used to increase efficiency for abstractors. For example, Flatiron Health has a technology to identify and highlight documents that are missing needed information. There are also “form validators to help prevent errors at time of entry,” tools to monitor performance and changes in documentation patterns so root causes can be addressed, and protocols for abstractors to escalate complex cases for clinical quality review.
The Cancer Surveillance Workforce
The Influence of Cancer Registry Professionals on Cancer Surveillance
Cancer registrars are data information specialists who capture and maintain a complete history, diagnosis, treatment, and health status for every person with cancer in the United States,101 said Nadine Walker, senior director of professional practice at NCRA.
___________________
98 See https://www.interfaceware.com/hl7-adt (accessed October 19, 2024).
99 See https://cynchealth.org (accessed October 19, 2024).
100 See https://www.crisphealth.org/about-crisp (accessed October 24, 2024).
101 See https://www.ncra-usa.org/About/Become-a-Cancer-Registrar/What-Do-Cancer-Registrars-Do# (accessed November 13, 2024).
Walker said that a key role of cancer registrars is to ensure the quality and validity of cancer surveillance data. Cancer registry data are used to “monitor and advance cancer treatments, conduct research, and improve cancer prevention and screening efforts” (NCRA, 2024a). Registrars enter data in accordance with numerous rules, standards, and guidelines, and the process is time intensive. As an example, Walker said that if the AJCC stage is not in the patient record, the registrar uses their expertise to discern this information from the medical record for abstracting.
Founded in 1974, NCRA is a membership organization whose mission is “to empower and advance registry professionals through innovations in education, advocacy, credentialing, and strategic partnerships,” Walker said (NCRA, 2024b), which includes working with standards-setting organizations to improve data standardization. NCRA credentialing of registrars began in 1983. The Oncology Data Specialist (ODS) credential102 is nationally recognized and “demonstrates competency and a clear understanding of the application of coding rules and the guidelines needed to capture and manage clinical cancer data,” she said. NCRA supports many opportunities for ongoing training and professional development through its Center for Cancer Registry Education103 and conferences, workshops, and publications.
Walker shared data from NCRA’s most recent workforce study,104 which found that 95 percent of the cancer registry workforce is satisfied with the profession, but nearly 20 percent plan to retire or leave in the next 5 years. Walker suggested several reasons cancer registrars might leave the profession, including difficulty getting employer support for education and a lack of respect in many health care settings, which she said was due to a lack of awareness about their important role in cancer control efforts.
As the field of cancer surveillance evolves, “a well-educated and certified cancer registry workforce will be needed to verify that cancer data collected through artificial intelligence or other technological advances is complete and accurate,” Walker concluded.
Vision for the 21st-Century Cancer Surveillance Workforce
Kevin Ward, director of the Georgia Center for Cancer Statistics105 at the Rollins School of Public Health at Emory University, discussed pathology as a use case for envisioning a transforming workforce. In all U.S. states
___________________
102 See https://www.ncra-usa.org/ODS-Credential (accessed October 24, 2024).
103 See https://www.cancerregistryeducation.org (accessed October 24, 2024).
104 See https://www.ncra-usa.org/Portals/68/PDFs/Workforce/WorkloadExecutiveSummary_2024.pdf (accessed October 24, 2024).
105 See https://sph.emory.edu/GCCS/gcr/intro.html (accessed October 24, 2024).
and territories, cancer is a reportable disease. In Georgia it is reported to the Georgia Department of Public Health, and the law106 allows the health department to make policies accordingly. Ward explained that this means that implementing new data collection practices, such as collecting recurrence data, can be done through policy change and not necessarily legislative action.
Ward said pathology data elements are critical for population-based registries, second only to the data in the NAACCR record layout.107 Ninety-four percent of all cancer cases in the United States have microscopic confirmation. However, he noted that pathology data are often lacking for brain tumors (which are frequently confirmed by radiology) and highly fatal cancers. He concurred with Charlton that the timely capture of every case of cancer in the state is necessary for surveillance and to support research. Fourteen federally funded R01108 research studies in Georgia are using registry data because, he said, the law allows access to the data and the ability to invite patients to participate in studies. Having timely pathology data facilitates rapid case ascertainment and identification of patients who might be suitable for a particular study (Petkov et al., 2024; Tucker et al., 2019). He added that “the majority of people want to participate in cancer research studies.”
The current pathology workflow, Ward summarized, spans real-time data generation by laboratories; standardized messaging; screening for reportability using tools such as eMaRC Lite and transmission to registries; autocoding, which can enable more rapid identification of patients; and production of reports by registries.
Data collected by facilities that provide care are reported to registries in electronic format, Ward said, and central registries merge multiple reports for the same patient into a single record. At the hospital level, the model is for an ODS-Certified cancer registrar to abstract EHR and pathology data for entry into the registry software. As the field of cancer registration transitions to new data models, Ward said, a shift in workforce roles will also be needed. The ODS-Certified cancer registrar, as the expert on cancer data, will work collaboratively with IT staff and vendors to integrate EHR and pathology data into registry software, he said. With less manual abstraction needed, the ODS-Certified cancer registrar’s work will shift to quality control of the data. Importantly, they will continue to be involved in the abstraction of complex data elements and in decision making (e.g., abstracting multiple primary cancers).
___________________
106 See https://dph.georgia.gov/chronic-disease-prevention/georgia-comprehensive-cancer-registry/reporting-cancer (accessed October 24, 2024).
107 See https://apps.naaccr.org/data-dictionary/data-dictionary/version=25/chapter-view/record-layout-table (accessed October 24, 2024).
108 See https://grants.nih.gov/funding/activity-codes/R01 (accessed October 19, 2024).
Castellanos agreed with Ward’s vision of optimizing the human workforce to support evolving surveillance models that leverage technology. She suggested looking at the ways data entry could be done at the point of care, ensuring that any approaches fit into the clinician workflow. Consideration could also be given to the different ways clinicians capture information in routine practice, from paper records to smart devices. Attention is also needed on the other end of the pathway, increasing the efficiency of the curation of nonstructured and complex data and determining the most effective way to deploy people in this process.
Gerhart suggested that there is a role for patients in the collection of cancer data. She said prompts in the portal can directly ask patients for information about social determinants of health, their cancer care experience, and their goals.
Recruiting and Retaining the Cancer Surveillance Workforce
Multiple participants shared their perspectives on challenges and opportunities for growing the pipeline of registry professionals and supporting those in the profession.
Walker said NCRA currently accredits 27 formal education programs in cancer registry management109 and has developed a textbook on cancer registry management.110 It also works to increase awareness of the profession by reaching out through Health Information Management programs, inviting students to the annual NCRA conference, and conducting outreach at the high school level. Walker referred participants to the NCRA website for more information.111 Charlton said the Iowa Cancer Registry’s annual Cancer in Iowa report112 receives a lot of news coverage in the state, and the recent report included an interview with an ODS-Certified cancer registrar highlighting the profession. The registry also works with training programs at the local community college. One challenge, she said, is getting some of the hospitals in Iowa to take students, and she emphasized that if hospitals do not participate in ODS-Certification training, then trained professionals will not be available when needed. Kohler said NAACCR collaborated with
___________________
109 See https://www.ncra-usa.org/Education/Accredited-Education-Programs-Old/Associate-and-Certificate-Programs (accessed October 24, 2024).
110 See https://www.ncra-usa.org/About/Store/4th-Edition-Textbook (accessed October 24, 2024).
111 See https://www.ncra-usa.org/About/Become-a-Cancer-Registrar (accessed October 8, 2024).
112 See https://shri.public-health.uiowa.edu/cancer-data/reports/iowa-cancer-reports/ (accessed October 19, 2024).
NCRA and Rutgers University to institute a certificate program for undergraduate students in public health.113 She noted the significant interest in the program, with 60 students enrolling in the first session. Ward and Charlton discussed the need to start early and raise awareness of the profession among high school students. Walker agreed and reiterated that NCRA does outreach to high schools, and she clarified that an associate’s degree is a requirement for pursuing ODS certification.114
Ward said that Georgia offers opportunities for some ODS-Certified staff to engage in research activities. Castellanos said appropriate compensation is also important, and Ward added that NCRA conducts salary surveys and noted that salaries for ODS staff are increasing.
Spencer highlighted the need to prepare registrars for their future roles as the workflow evolves. Winn observed that technology is evolving at a rapid pace and said it is important to consider how a variety of educational pathways might enhance the cancer surveillance workforce. Palis suggested that the role of the registrar could evolve beyond data entry and data quality to being “at the center of patient quality of care.” He said that registrars at CoC-accredited hospitals are involved in “monitoring compliance with key metrics” and discuss reports at cancer committee meetings “down to the case level.” One workshop participant suggested that the registry profession needs to be marketed to those with interest and expertise in data analytics. An ODS-Certified cancer registrar not only collects data but can understand and present them to the hospital administration in the context of monitoring patient quality of care.
Several session panelists also discussed promoting ODS-Certified careers to nurses. Ward said some of the most successful ODS-Certified staff are nurses because of their medical and health care background. Walker confirmed that NCRA does see many nurses transition to the profession and agreed with the need to raise awareness about the profession broadly among health care professionals.
Lori Swain, executive director of NCRA, said NCRA is working with the Bureau of Labor and Statistics to develop a definition of medical registrar in anticipation of the 2028 revisions to the Standard Occupational Classification.115 She noted that registrars are currently classed with health information technicians, but before 2017, registrars were classed with medical records specialists.
___________________
113 To learn more about the Cancer Surveillance and Epidemiology Certificate for undergraduate students at Rutgers University, see https://bloustein.rutgers.edu/undergraduate/#certificates (accessed November 25, 2024).
114 See https://www.ncra-usa.org/ODS-Credential/Potential-ODS/Eligibility (accessed October 24, 2024).
115 See https://www.bls.gov/soc (accessed October 19, 2024).
POLICY OPPORTUNITIES FOR ADVANCING PROGRESS IN CANCER SURVEILLANCE
Many speakers from federal agencies and other interested parties shared their perspectives on policy opportunities for advancing progress in cancer surveillance and achieving the vision for the future. Penberthy and Cathy Bradley, dean of the Colorado School of Public Health and deputy director of the University of Colorado Cancer Center, discussed the need to identify priorities and whether an existing body could facilitate this discussion with key collaborators.
Improving Reporting of Childhood Cancer Data
Every year, about 15,000 U.S. children under the age of 20 are diagnosed with cancer,116 Jones said. She never thought her child, Noah, would be one of them (see Box 3). Jones stressed the importance of cancer surveillance for improving patient care across the cancer continuum and explained that data from studies of treatments in adults do not necessarily translate to children. Real-time data are needed to inform timely treatment decisions and, importantly, “faster reporting of childhood cancer data can help researchers design clinical trials, share data, and identify pediatric patients for clinical trials sooner,” Jones said. She emphasized the critical importance of providing families with information on potential clinical trials as soon as a diagnosis is made, before any treatment starts, which could lead to eligibility for a clinical trial. Jones said that many clinicians Noah saw during his treatment were often located at different facilities, with data systems that were not interoperable.117 She highlighted CDC’s ongoing efforts to improve timely reporting and facilitate sharing of standardized data, including daily pathology reporting leveraging the APHL-AIMS platform, TEFCA and QHINs, and NPCR NOAH (named in honor of her son). She also emphasized the need to educate the public about the importance of sharing patient experiences and data to help other patients.
Spencer asked about the status of real-time connections between registries and research, particularly for pediatric cancers. Penberthy said the NCI
___________________
116 See https://www.cdc.gov/national-program-cancer-registries/about/pediatric-young-adult-cancer.html (accessed November 13, 2024).
117 This included a pediatrician, radiologist, orthopedic surgeon, neurologist, emergency room physicians, hospitalist, pathologists, medical oncologists, neurosurgeons, radiation oncologists, anesthesiologists, neuro-ophthalmologist, pediatric physiatrist, occupational speech therapist, robotics therapist, pharmacist, clinical trial investigator and coordinator, palliative care oncologist, social worker, and hospice nurses.
National Childhood Cancer Registry (NCCR)118 is being developed with funding from the Childhood Cancer Data Initiative.119 NCCR is drawing data from SEER registries and developing linkages to NPCR registries and other data sources, such as claims data, pharmacy data, and the Children’s Oncology Group.120 NCCR is also working to implement ExtractEHR. Penberthy said, “there is a very robust authentication and authorization process” to access
___________________
118 See https://cancercontrol.cancer.gov/research-emphasis/supplement/childhood-cancer-registry (accessed October 19, 2024).
119 See https://www.cancer.gov/research/areas/childhood/childhood-cancer-data-initiative (accessed October 19, 2024).
120 See https://www.childrensoncologygroup.org/about (accessed October 19, 2024).
NCCR data for research. She added that, because pediatric cancers are so rare, geographic data are never released with any datasets to reduce the risk of patient reidentification.
Improving Return on Investment for Cancer Registries
Penberthy discussed several adaptations that could increase the return on investment for cancer registries. One area of focus for SEER is reducing the 2-year lag time from diagnosis to reporting121 through electronic pathology reporting. SEER is also working on establishing novel linkages to incorporate key clinical variables (e.g., biomarkers, genetic data, treatment, and outcomes, such as recurrence).
Another approach is increasing the research return on investment through “out-linkages” that facilitate more widespread use of SEER data for research by other systems. For example, Penberthy said a two-way data linkage between SEER and the Veterans Health Administration (VHA) Central Cancer Registry ensures that all VHA Central Cancer Registry patients are in SEER and that the VHA has data on all veterans in SEER.122 SEER is also planning to share de-identified registry data with Myriad Genetics123 to provide clinical context for its genomic and genetic testing data (e.g., outcomes and stage at diagnosis) and developing similar arrangements with other genetic testing laboratories and the All of Us Research Program.124
SEER supports population-level analyses of key quality indicators. Examples discussed by Penberthy included “benchmarking the quality of care at the population level for BRCA125 testing in ovarian cancer, … monitoring trends in dissemination of new treatments or new diagnostic methods, … providing population-level data to help identify high-risk populations, … [and] providing real-time access … to rapidly identify patients who might be eligible for studies.”
Penberthy shared her suggestions for policy changes that could improve cancer surveillance. First, she said, “mandated electronic pathology report-
___________________
121 See https://surveillance.cancer.gov/delay (accessed October 24, 2024).
122 See https://news.va.gov/press-room/va-and-national-cancer-institute-announce-historic-data-sharing-partnership-to-better-understand-and-treat-cancer-among-veterans (accessed October 24, 2024).
123 See https://myriad.com/about-myriad-genetics (accessed October 19, 2024).
124 See https://allofus.nih.govs (accessed October 19, 2024).
125 BReast CAncer gene, better known as BRCA, is a group of genes that code for DNA repair proteins. Mutations convey a higher risk for cancer, especially breast and ovarian cancers. See https://www.cancer.gov/about-cancer/causes-prevention/genetics/brca-fact-sheet (accessed October 19, 2024).
ing in every state” would facilitate real-time reporting and the assessment of patients for potential enrollment in clinical trials. “Mandated authorization for registries to access all payer claims data” would enable capture of data on longitudinal treatment, recurrence, and comorbid conditions, she continued. Penberthy concluded by urging CMS to share claims data for surveillance and incorporation into the registry and mandate recurrence data reporting.
Resource Needs for High-Quality Cancer Surveillance
Micky Tripathi, assistant secretary for technology policy at ONC, provided an overview of current and future ONC activities related to cancer surveillance. ONC has been working with the National Institutes of Health (NIH), NCI, the White House Cancer Moonshot Team, and other partners to improve the availability of data, both bringing data into the EHR and sharing it for authorized uses.
Tripathi said 97 percent of hospitals and 80 percent of clinician offices in the United States use ONC-certified EHR systems, which support standardization and data availability.126 ONC maintains USCDI (the minimum dataset required for EHR systems) and has been working with public and private partners to develop domain-specific extensions, including USCDI+ for Cancer, which will support the data requirements of the CMS Enhancing Oncology Model127 and clinical trial matching.
As directed by the 21st Century Cures Act,128 ONC implemented TEFCA in late 2023 to support “nationwide network-to-network interoperability,” Tripathi said, and there are currently seven TEFCA QHINs129 that are sharing clinical data for treatment purposes. The next step in TEFCA implementation is to support the nationwide exchange of clinical information for research purposes, which Tripathi explained has additional complexities. Challenges to be addressed include standardized consent (in a computable format usable across EHR systems) and standards for data de-identification. ONC has also been working with payers to facilitate clinician access to claims data via TEFCA, in line with the requirements of the CMS interoperability rule.
Tripathi said there will be continuing need for intermediary data curation services, such as registries, so that data generated for health care delivery can
___________________
126 See https://www.healthit.gov/buzz-blog/health-it/oncs-next-chapter (accessed October 19, 2024).
127 See https://www.cms.gov/priorities/innovation/innovation-models/enhancing-oncology-model (accessed October 19, 2024).
128 See https://www.fda.gov/regulatory-information/selected-amendments-fdc-act/21st-century-cures-act (accessed October 19, 2024).
129 See https://www.healthit.gov/sites/default/files/page/2023-11/TEFCA_2-Pager_Digital_508.pdf (accessed October 24, 2024).
be leveraged for other purposes, including research and public health needs. The goal is to use emerging tools to make the curation process “better, faster, and cheaper than it is today.”
The Need for Data to Assess the Quality Impact of Payment Models
“Value-based care ties the amount that health care providers earn for their services to the results they deliver for their patients,” said Nancy Keating, professor of health care policy and medicine at Harvard University (Lewis et al., 2023). She discussed the CMS Oncology Care Model (OCM)130 for chemotherapy administration as an example of how data are used in alternative payment models.
While OCM was in effect from 2016 through June 2022, more than 1.5 million patient episodes of care were completed across more than 200 participating practices, Keating said.131 She explained that practices could bill $160 per patient per month during a 6-month chemotherapy episode. Practices were also eligible for performance-based payments based on cost and quality of care. Keating described the OCM as “modestly successful from a financial standpoint, saving about $600 per episode on average across the whole performance period” (OCM Evaluation Team, 2024).
CMS evaluated the impact of the OCM on the quality of care across 12 measures in areas of performance-based payment, timeliness of chemotherapy, guideline recommended treatment, chemotherapy associated use, and end-of-life care. Keating reported that there was no evidence that OCM improved the quality of cancer care for any of the measures.132
Keating listed several areas of opportunity to advance progress. First, payment models focus on patients who receive treatment but do not provide insight into who is able to access treatment or whether patients are receiving appropriate guideline-based treatment. Second, these are payment models led by payers, and the data at their disposal are limited to submitted claims and data reports from practices in the model. She also noted the critical importance of having appropriate comparison groups to understand the quality impact of payment models. “Quality measurement in oncology care is early in its development,” Keating continued, and “claims-based measures identify a
___________________
130 See https://www.cms.gov/priorities/innovation/innovation-models/oncology-care (accessed November 3, 2024).
131 See https://www.cms.gov/priorities/innovation/data-and-reports/2023/ocm-evaluation-pp1-9 (accessed November 20, 2024).
132 See https://www.cms.gov/priorities/innovation/data-and-reports/2024/ocm-final-eval-report-2024-exec-sum (accessed October 8, 2024).
very limited aspect of quality.” Last, she said, “Real-time, high-quality registry data are needed to fully assess the impacts of payment models on quality.”
Advancing Data Linkages to Biospecimens to Enable Population-Based Studies
Michaela Dinan, associate professor of epidemiology at Yale University, said that to conduct population sciences research that explores the intersection between social and biological factors, it is essential to have population-level data with linkages to biological samples, but few if any such linkages exist. Dinan observed the “disconnect between population sciences and basic biology,” including how investigators identify themselves, the types of funding for research in each field, the research infrastructure needed, and their journal audiences. Furthermore, genomic datasets are often siloed, and privacy concerns impede sharing patient genetic data.
To address this challenge, Dinan and colleagues conducted a pilot feasibility study linking SEER data, Medicare claims data, and physical tumor samples to analyze socioeconomic, clinical, and genomic factors associated with mortality in women with newly diagnosed breast cancer (Robinson et al., 2021). She said they are now linking “SEER–Medicare data to select cohorts of patients at the population level … to detect rare phenotypes or outcomes of interest for further study.”
Dinan highlighted several opportunities to address policy barriers to population-level genomic analyses, particularly current restrictions on data sharing and use. To protect patient privacy, publication of individual-level data is prohibited per the data use agreement for the NCI SEER–Medicare data linkage.133 Derived data cannot be shared in a public data repository and must be destroyed after studies are completed, she explained. This is counter to the general practice of submitting generated genomic data to public repositories to enable “validation and replication of published research and re-use by other researchers for further discovery-based analysis of these unique and often costly cohorts,” she said.
The requirements of the NIH Genomic Data Sharing (GDS) policy134 also present challenges, Dinan said. It mandates that data from large-scale genomic research conducted with NIH funds must be shared, and consent for sharing is required from all patients (living and deceased). Dinan said, “obtaining consent at the population level makes these types of studies logistically impossible,” which essentially rules out using NIH funding for the study
___________________
133 See https://healthcaredelivery.cancer.gov/seermedicare/obtain/seerdua.pdf (accessed October 19, 2024).
134 See https://sharing.nih.gov/genomic-data-sharing-policy (accessed October 19, 2024).
of larger cohorts. She also pointed out that the GDS policy and the SEER–Medicare data use agreement are “in direct conflict” regarding the sharing of genomic data.
Another challenge, Dinan said, is that “not all SEER registries participate in these linkage programs,” and she noted that she was aware of seven SEER registries participating in a Virtual Tissue Repository program.135 In addition, she said, “logistical workflows to maintain de-identification of these data linkages are complex,” and there are significant costs associated with obtaining formalin-fixed, paraffin-embedded tissue samples.
Aligning Incentives to Improve Data Capture
Gerhart offered actions key collaborators can take now to drive improvement in data capture and enable improvements in cancer care, research, and patient experience.
Reiterating that data standards are essential for the exchange of health care data, Gerhart encouraged those in government to learn about TEFCA and advocate for organizations they work with to participate in TEFCA. She urged payers, and CMS in particular, to “measure and pay for what matters.” Metrics are often quantity based (e.g., number of cancer screens ordered), but there are other metrics of value to oncology clinicians and researchers that could be identified and implemented, and less relevant metrics could be eliminated.
Gerhart also reiterated the need for clinicians and health systems to embrace the use of structured data and highlighted the importance of taking an approach that incentivizes and rewards the uptake of structured data entry rather than a penalty-based approach. She said another potential action that can be taken now is to automate and simplify electronic synoptic pathology reporting. The technology exists, as do existing systems that can be leveraged, and vendors are ready to work with health systems and clinicians to improve these processes. Gerhart said technology companies could focus on ease of use in product development, such as developing simple, smart forms for data capture by the patient’s care team and cancer registrars. Vendors could also design products to “make the right thing to do the easy thing to do,” she said.
Advocates for improved cancer surveillance can bring interested parties together in interdisciplinary working sessions to share experiences and lessons learned and collaborate on solutions, Gerhart said. Advocacy efforts are also needed to show the value of cancer registries and registrars to policy makers and clinicians and market the ODS profession to grow the workforce.
___________________
135 See Sanchez et al., 2024.
Several participants discussed further some of the aspects of EHRs for registry data capture. Larissa Nekhlyudov, professor of medicine at the Brigham and Women’s Hospital at Harvard Medical School and clinical director of internal medicine for cancer survivors at the Dana-Farber Cancer Institute, emphasized that there are various nonstructured ways clinicians enter patients’ data, including dictation. Gerhart said that technologies such as optical character recognition can help facilitate the conversion of unstructured data to structured, and Spencer mentioned voice-enabled dictation as a way to insert information into a structured, synoptic form. Nekhlyudov stated that EHRs are also being used as a tool for communication with patients (Singh et al., 2024). Gerhart agreed and said that “less is more,” emphasizing that EHR documentation, such as clinical notes, should only contain information that matters to the patient and clinicians. She added that data in the EHR should provide a better view of where the patient is in their cancer care (which is often not apparent from the information in the record of an encounter). Gerhart discussed that Epic EHR software is customized by each health system according to its needs. Gerhart said Epic focuses on implementing standards and structures that are broadly applicable, and she reiterated TEFCA as an example. Gerhart also noted the potential for data in EHRs to power research on rare diseases.
Policy Opportunities from Technological Innovations
Andrew Hantel, faculty member of the Divisions of Leukemia and Population Sciences at the Dana-Farber Cancer Institute and assistant professor of medicine at Harvard Medical School, shared his perspective as a registry user on policy actions related to technology innovations.
Diagnostic and surveillance technologies are advancing rapidly, Hantel said, from liquid biopsies to multicancer detection 136 and patient-facing technologies, such as wearables and emerging generative AI tools. He suggested that there is “an opportunity to link novel device development and registries much earlier, rather than relying on post-marketing real-world evidence studies.” He also suggested that the registry and surveillance community should provide input into ongoing efforts to update standards of device regulation, particularly concerning new AI-based devices that fall outside the traditional device regulation pathways of the U.S. Food and Drug Administration. He suggested that this “merger of registry and device policy” could help to overcome some of the challenges discussed. Also needed are policies addressing
___________________
136 Multicancer detection tests are blood tests that screen for multiple cancer types through the presence of certain biomarkers. See https://www.cancer.gov/publications/dictionaries/cancer-terms/def/mcd-test (accessed December 17, 2024).
the existing infrastructures that use novel technologies (e.g., surveys, bioassays, and wearables) to capture other types of population data, such as the All of Us program, Hantel said. He noted that “opt-in datasets” can exacerbate representation biases but added that recent opt-in programs have made strides to minimize this issue through community engagement and stratified sampling, such as in the All of Us program.
Hantel also discussed the need to develop communications policies that more effectively disseminate registry-related information to target audiences, including the public, local legislators, and funders. Registry data are routinely shared via publication in medical journals and public health initiatives, but even public reports on cancer registry websites can be very technical. Communications policies are needed to leverage technology for multimodal storytelling through social media and interactive registry website content for lay audiences. Hantel suggested that a public educated about the benefits of cancer surveillance programs can be advocates or “cancer registry influencers” for funding allocation and local legislation.
Policy Opportunities to Improve Sharing of Patient Data
Deven McGraw, chief regulatory and privacy officer at Ciitizen,137 discussed policy opportunities to improve data sharing from a privacy and data governance perspective. She expanded on the concept raised earlier of a national patient identifier to better facilitate data linkages. Creating such an identifier was authorized under HIPAA in 1996. However, every year since, the Health and Human Services (HHS) Appropriations Bill has included language prohibiting HHS from funding or acting on this authorization. Policy advocacy is needed to demonstrate the importance of a national patient identifier for patient care, registry and surveillance efforts, and research, she said, and show that data sharing can occur while preserving patient privacy.
The impact of state policies on data sharing by registries was also raised earlier, and McGraw pointed out that state legislators meet infrequently and often do not receive sufficient education about measures under consideration because of the time constraints of their sessions. She supported the idea of developing model legislation for states that would allow data sharing by registries while protecting patient privacy.
McGraw said that patient organizations are starting to proactively develop registries and fund research directly.138 She noted that provisions of the 21st Century Cures Act, particularly the prohibitions on information blocking, and
___________________
137 See https://www.citizen.health/about (accessed October 24, 2024).
138 See https://www.iqvia.com/newsroom/2023/10/patient-organizations-gave-22-billion-in-grants-for-research-patient-services-and-access-to-care-ove (accessed October 8, 2024).
the tools being developed by ONC are helping to enable these efforts. As an example, McGraw mentioned how Ciitizen “worked with the Leukemia and Lymphoma Society139 to quickly power a study on the efficacy of COVID-19 vaccines in their patient community” (Greenberger et al., 2021). Ciitizen has also worked with the Cholangiocarcinoma Foundation140 to enable its research registry, which matches patients with studies. Patients want to be “part of the solution, and this is one way to do that,” she said.
Ethics, Privacy, and Cancer Registries
Aaron Goldenberg, professor and vice chair in the Department of Bioethics at Case Western Reserve University, discussed contextual and social distinctions that influence the development of ethical policy and data governance for cancer registries. Contextual distinctions include the needs of individuals versus populations; whether the data in question are identified or identifiable versus de-identified or anonymized; and whether goals of data access are for clinical, quality improvement, or quality assurance versus research use. Social distinctions include the potential for physical and practical harms or benefits versus dignitary harms or benefits; consent versus data security; and being trusted by communities versus being trustworthy in the eyes of the community. However, “these are all false dichotomies,” Goldenberg said. He suggested that the distinction between patient privacy and data access discussed throughout the workshop is also a false dichotomy.
Goldenberg proposed an approach to developing cancer registry policies that address the challenges facing different populations and focus on the foundational principles of engagement and ethical stewardship. He explained that bioethics centers around patient and community values. Cancer registry policies need to reflect those values “in much more holistic ways than they currently do,” and he emphasized the need to be proactive in engaging legislators and ensuring they take patient values into account in policy development. Approaches could include providing model legislation to states or conducting legislative workshops. It is possible to develop policies that both maximize patient privacy and facilitate data access, he said.
As discussed, Goldenberg said patients are generally supportive of their data being used. However, it is important to acknowledge the concerns some communities have regarding the use of their data and ensure that their values and concerns are reflected in the policies developed. Based on his research, Goldenberg said that patient willingness to participate in registries and biorepositories is associated with the extent to which the research done using their
___________________
139 See https://www.lls.org (accessed October 19, 2024).
140 See https://cholangiocarcinoma.org (accessed October 19, 2024).
data will be relevant to and bring benefit to their community (Goldenberg et al., 2011). Recent interviews with biobank leaders highlighted the importance of ethical stewardship of patient samples and data. Many registries emphasized their responsibility to serve as honest brokers of patient information and to engage patients as partners in the research use of their data. Goldenberg added that, for many biobank leaders, concerns about ethical stewardship also included ensuring the preservation and protection of data if a registry shuts down (Cadigan et al., 2024). He noted a policy opportunity to address “the lack of policies and governance structures that allow for sustainability if grant funding ends.”
REFLECTIONS
In the final session, moderators from the prior sessions highlighted observations made throughout the workshop and summarized opportunities discussed to advance progress in cancer surveillance based on speakers’ remarks (see Boxes 1 and 2). The data collection challenges experienced by registries were a recurring theme, Nadia Howlader, program director in the Statistical Research & Applications Branch of the Surveillance Research Program at NCI, said. These included difficulties in capturing comprehensive, real-world cancer data due to rapid advancements in treatment, variability in data sources, and limitations in current reporting systems. Many participants discussed motivating the submission of complete and accurate cancer data by linking reimbursement to cancer reporting.
Other approaches to improving data collection that were discussed included developing and promoting the use of standardized reporting protocols to improve consistency and accuracy of reporting across health care facilities and providing the training and resources needed for clinicians and others to comply with new reporting requirements, Howlader reported. Several participants suggested using registries to gather select data (e.g., recurrence and treatment) from a population-representative sample to support inferences about the total population.
Another main topic across the workshop was the need for improved data integration and interoperability of data sources, Richardson said. Many speakers discussed establishing national standards for data exchange; partnering across government, health care organizations, and technology vendors to foster widespread adoption of standards and platforms; and providing the funding and technical support needed to support upgrading of data systems and compliance with new standards, Richardson summarized.
Nekhlyudov highlighted the significant impact that state and governmental regulatory policies have on data access, linkages, and sharing. Multiple participants discussed revising state policies to facilitate data access, linkages, and sharing while still protecting patient privacy, Gomez said. Approaches
suggested by several speakers included leveraging privacy-preserving record linkages, federated data query models, the NAACCR Virtual Pooled Registry Cancer Linkage System,141 and partnering with patient advocates to raise awareness of the importance of registry data, she said.
Many speakers representing registries and registry data users described other challenges, such as level or reduced funding in the face of rising costs for cancer surveillance; delays in data availability for reporting and research; data gaps, particularly for recurrence and disease progression; challenges linking to other data sources; a lack of denominator data for analysis of trends; and balancing the need for data granularity with preserving patient privacy in small populations, Nekhlyudov summarized. Gomez said that, in considering how to capture more timely and complete surveillance data, many participants noted the need for a multipronged approach that incorporated human review. Suggestions included creating efficient data linkages to fill gaps (including patient surveys) and increasing funding to support the implementation of improved registry infrastructure and advanced technologies.
Yu summarized the range of methodological and technological challenges to be addressed, for example, data are frequently not in a structured format and require human curation; critical data are often missing; registry data are not available as timely as is needed; harmonized standards to enable data interoperability and sharing are lacking; use cases are needed to establish what data are really needed; the ideal balance across human curation, structured data capture, and generative AI is still to be defined; clinicians already are overburdened by documentation; and current payment models do not incentivize quality data capture. The need to develop the business case for organizational investment in infrastructure to support data entry, extraction, and registry population was also discussed, Yu said.
Shulman said that increased incentives will be needed to ensure structured entry of critical data. The challenge is to increase data timeliness and granularity without increasing the burden on clinicians and cancer registrars, he said. Several speakers discussed the need for federal agencies to align around data standards and data sharing policies and the need to harmonize state-level policies that impact data sharing and use and take a fresh look at data privacy laws that were passed decades ago, Shulman summarized. Many speakers also noted the importance of establishing public and private partnerships in addressing these challenges.
Regarding infrastructure and the evolving cancer surveillance workforce, several panelists discussed improving the pathology workflow and patient outcomes by using the CAP Cancer Protocols and leveraging opportunities for data automation, Palis said. Richardson highlighted several of the opportuni-
___________________
141 See https://apps.naaccr.org/vpr-cls (accessed December 18, 2024).
ties to advance progress noted by many speakers, including providing continuing education regarding the evolving landscape of data capture; recruitment and retention of ODS-Certified registry staff, marketing the profession to highlight the role of the ODS-Certified professional in data analytics; developing tools to assist registries with case finding to enable timelier reporting; and leveraging linkages to EHR data to provide a more complete picture of the patient’s cancer experience. A recurring theme mentioned by many participants was the need to ensure that every person with cancer is counted, Richardson said.
Bradley recapped other key issues identified by speakers. Multiple types and sources of cancer data exist, data capture and curation are labor intensive, and efforts are often duplicative, she said. Multiple panelists highlighted the need for real-time, high-quality registry data to support quality improvement and evaluation of care and payment models and inform timely treatment decisions by patients and families. Additional aspects of data linkages discussed included the costs of maintaining and updating them and the need for patient identifiers that could more readily facilitate linking patient data. Several participants raised the issue of sustainability of registries and legacy biospecimens, given the funding challenges facing some registries and tissue banks. The topic of false dichotomies that influence the development of ethical registry policies and data governance was also discussed.
Yabroff highlighted other policy opportunities discussed throughout the workshop. These included suggestions by some participants for mandated reporting of electronic cancer pathology data; funding of collaborations between registries, EHR vendors, and other partners to develop multipronged approaches for addressing data gaps (e.g., synoptic reporting and payor mandates); developing model legislation for states that enables data linkages and sharing and addresses NIH policy barriers to data sharing; and developing workforce education and training that incorporates “both high tech and high touch.”
There was also discussion of partnering with advocates to raise awareness of the need to allocate federal funding for a national patient identifier and educate the public and policy makers about the value of cancer registries, cancer surveillance, and sharing of cancer data in general. Nekhlyudov suggested the need to engage professional organizations to better inform clinicians.
In closing, Richardson said, “population-based cancer registries are a public good” and reiterated the need to remember that every data point in a registry represents a patient’s journey.
REFERENCES
Abrahão, R., A. Brunson, J. Chubak, K. J. Wernli, H. B. Nichols, C. Chao, K. J. Ruddy, E. E. Hahn, Q. Li, M. H. Malogolowkin, C. A. M. Sauder, L. H. Kushi, T. Wun, and T. H. M. Keegan. 2024. Late venous thromboembolism in survivors of adolescent and young adult cancer: A population-based study in California. Thrombosis Research 235:1–7.
Acosta, J. N., G. J. Falcone, P. Rajpurkar, and E. J. Topol. 2022. Multimodal biomedical AI. Nature Medicine 28(9):1773–1784.
ACS (American College of Surgeons). 2022. National Cancer Database Tools. https://www.facs.org/media/buvfsm0p/22_ca_ncdbtools_8-5x11_v01.pdf (accessed October 23, 2024).
Arnaout, A., S. Mukhi, J. Brehaut, S. Davidson, M. Fung Kee Fung, P. Hebbard, C. Hillis, R. Leonard, L. A. Mack, A. Mathieson, J. Presseau, D. Schaeffer, A. Seely, G. Stuart, M. Tesch, N. Westhuizen, and C. Earle. 2024. Implementation and evaluation of a national quality improvement initiative in cancer surgery. BMJ Open Quality 13(2).
Arndt, V., and S. Siesling. 2024. Krebsregistrierung in Europa. Die Onkologie 30(4):245–256.
CPAC (Canadian Partnership Against Cancer). 2018. The 2018 Cancer System Performance Report. Toronto (ON): Canadian Partnership Against Cancer. https://s22457.pcdn.co/wp-content/uploads/2019/01/2018-Cancer-System-Performance-Report-EN.pdf (accessed October 16, 2024).
CPAC. 2024. Mandate. https://www.partnershipagainstcancer.ca/about-us/what-we-do/mandate/ (accessed October 16, 2024).
Cadigan, R. J., R. Ponsaran, C. Rich, J. Timmons, K. B. Brothers, and A. J. Goldenberg. 2024. Supporting stewardship: Funding, utilization, and sustainability as ethical concerns in networked biobanking. AJOB Empirical Bioethics:1–10.
Castellanos, E. H., B. L. Wittmershaus, and S. Chandwani. 2024. Raising the bar for real-world data in oncology: Approaches to quality across multiple dimensions. JCO Clinical Cancer Informatics 8:e2300046.
CCC19 (COVID-19 and Cancer Consortium). 2020. A systematic framework to rapidly obtain data on patients with cancer and COVID-19: CCC19 governance, protocol, and quality assurance. Cancer Cell 38(6):761–766.
Charlton, M. E., A. R. Kahl, B. D. McDowell, R. S. Miller, G. Komatsoulis, J. E. Koskimaki, D. R. Rivera, and K. A. Cronin. 2022. Cancer registry data linkage of electronic health record data from ASCO’s CancerLinQ: Evaluation of advantages, limitations, and lessons learned. JCO Clinical Cancer Informatics 6:e2100149.
Delude, C. M. 2015. Deep phenotyping: The details of disease. Nature 527(7576):S14–S15.
Gabriel, P. E., A. P. Singh, and L. N. Shulman. 2023. Re-envisioning the paradigm for oncology electronic health record documentation by paying for what matters for patients, quality, and research. JAMA Oncology 9(3):299–300.
Giannakeas, V., D. W. Lim, and S. A. Narod. 2024. Bilateral mastectomy and breast cancer mortality. JAMA Oncology Jul 25:e242212.
Goldenberg, A. J., S. C. Hull, B. S. Wilfond, and R. R. Sharp. 2011. Patient perspectives on group benefits and harms in genetic research. Public Health Genomics 14(3):135–142.
Greenberger, L. M., L. A. Saltzman, J. W. Senefeld, P. W. Johnson, L. J. DeGennaro, and G. L. Nichols. 2021. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell 39(8):1031–1033.
Grivas, P., A. R. Khaki, T. M. Wise-Draper, B. French, C. Hennessy, C. Y. Hsu, Y. Shyr, X. Li, T. K. Choueiri, C. A. Painter, S. Peters, B. I. Rini, M. A. Thompson, S. Mishra, D. R. Rivera, J. D. Acoba, M. Z. Abidi, Z. Bakouny, B. Bashir, T. Bekaii-Saab, S. Berg, E. H. Bernicker, M. A. Bilen, P. Bindal, R. Bishnoi, N. Bouganim, D. W. Bowles, A. Cabal, P. F. Caimi, D. D. Chism, J. Crowell, C. Curran, A. Desai, B. Dixon, D. B. Doroshow, E. B. Durbin, A. Elkrief, D. Farmakiotis, A. Fazio, L. A. Fecher, D. B. Flora, C. R. Friese, J. Fu, S. M. Gadgeel, M. D. Galsky, D. M. Gill, M. J. Glover, S. Goyal, P. Grover, S. Gulati, S. Gupta, S. Halabi, T. R. Halfdanarson, B. Halmos, D. J. Hausrath, J. E. Hawley, E. Hsu, M. Huynh-Le, C. Hwang, C. Jani, A. Jayaraj, D. B. Johnson, A. Kasi, H. Khan, V. S. Koshkin, N. M. Kuderer, D. H. Kwon, P. E. Lammers, A. Li, A. Loaiza-Bonilla, C. A. Low, M. B. Lustberg, G. H. Lyman, R. R. McKay, C. McNair, H. Menon, R. A. Mesa, V. Mico, D. Mundt, G. Nagaraj, E. S. Nakasone, J. Nakayama, A. Nizam, N. L. Nock, C. Park, J. M. Patel, K. G. Patel, P. Peddi, N. A. Pennell, A. J. Piper-Vallillo, M. Puc, D. Ravindranathan, M. E. Reeves, D. Y. Reuben, L. Rosenstein, R. P. Rosovsky, S. M. Rubinstein, M. Salazar, A. L. Schmidt, G. K. Schwartz, M. R. Shah, S. A. Shah, C. Shah, J. A. Shaya, S. R. K. Singh, M. Smits, K. E. Stockerl-Goldstein, D. G. Stover, M. Streckfuss, S. Subbiah, L. Tachiki, E. Tadesse, A. Thakkar, M. D. Tucker, A. K. Verma, D. C. Vinh, M. Weiss, J. T. Wu, E. Wulff-Burchfield, Z. Xie, P. P. Yu, T. Zhang, A. Y. Zhou, H. Zhu, L. Zubiri, D. P. Shah, J. L. Warner, and G. Lopes. 2021. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: A report from the COVID-19 and Cancer Consortium. Annals of Oncology 32(6):787–800.
Guadamuz, J. S., X. Wang, C. A. Ryals, R. A. Miksad, J. Snider, J. Walters, and G. S. Calip. 2023. Socioeconomic status and inequities in treatment initiation and survival among patients with cancer, 2011-2022. JNCI Cancer Spectrum 7(5).
Hawley, J. E., T. Sun, D. D. Chism, N. Duma, J. C. Fu, N. T. N. Gatson, S. Mishra, R. H. Nguyen, S. A. Reid, O. K. Serrano, S. R. K. Singh, N. K. Venepalli, Z. Bakouny, B. Bashir, M. A. Bilen, P. F. Caimi, T. K. Choueiri, S. J. Dawsey, L. A. Fecher, D. B. Flora, C. R. Friese, M. J. Glover, C. J. Gonzalez, S. Goyal, T. R. Halfdanarson, D. L. Hershman, H. Khan, C. Labaki, M. A. Lewis, R. R. McKay, I. Messing, N. A. Pennell, M. Puc, D. Ravindranathan, T. D. Rhodes, A. V. Rivera, J. Roller, G. K. Schwartz, S. A. Shah, J. A. Shaya, M. Streckfuss, M. A. Thompson, E. M. Wulff-Burchfield, Z. Xie, P. P. Yu, J. L. Warner, D. P. Shah, B. French, C. Hwang, and COVID-19 and Cancer Consortium (CCC19). 2022. Assessment of regional variability in COVID-19 outcomes among patients with cancer in the United States. JAMA Network Open 5(1):e2142046.
Hill, S. 2023. Mid-Level Tactical Group Reviews Change Requests to Data Standards. https://narrative.naaccr.org/mltg-v24_changes/ (accessed October 9, 2024).
Holleczek, B., A. Nennecke, B. Fell, and F. Peters. 2023. Follow-up information of comprehensive cancer registries in Germany: Valuable data with a solid evidence base. Die Onkologie 30:273–279.
Howell, M. D., G. S. Corrado, and K. B. DeSalvo. 2024. Three epochs of artificial intelligence in health care. JAMA 331(3):242–244.
Howlader, N., G. Forjaz, M. J. Mooradian, R. Meza, C. Y. Kong, K. A. Cronin, A. B. Mariotto, D. R. Lowy, and E. J. Feuer. 2020. The effect of advances in lung-cancer treatment on population mortality. New England Journal of Medicine 383(7):640–649.
Hsu, E., H. Hanson, L. Coyle, J. Stevens, G. Tourassi, and L. Penberthy. 2024. Machine learning and deep learning tools for the automated capture of cancer surveillance data. JNCI Monographs 2024(65):145–151.
In, H., I. Solsky, C. A. Simon, and D. P. Winchester. 2019. Lack of cancer recurrence data in large databases: A national survey of hospital cancer registries. Journal of Surgical Research 235:551–559.
Iyer, H. S., X. Shi, J. M. Satagopan, I. Cheng, C. Roscoe, R. H. McLaughlin, A. M. Stroup, S. Setoguchi, E. V. Bandera, B. Y. Hernandez, J. A. Doherty, M. C. Hsieh, R. Knowlton, B. Qin, F. Laden, T. R. Rebbeck, and S. L. Gomez. 2023. Advancing social and environmental research in cancer registries using geomasking for address-level data. Cancer Epidemiology, Biomarkers & Prevention 32(11):1485–1489.
Jones, D. E., T. O. Alimi, P. Pordell, F. K. Tangka, W. Blumenthal, S. F. Jones, J. D. Rogers, V. B. Benard, and L. C. Richardson. 2021. Pursuing data modernization in cancer surveillance by developing a cloud-based computing platform: Real-time cancer case collection. JCO Clinical Cancer Informatics 5:24–29.
Katalinic, A., M. Halber, M. Meyer, M. Pflüger, A. Eberle, A. Nennecke, S. Z. Kim-Wanner, T. Hartz, K. Weitmann, A. Stang, C. Justenhoven, B. Holleczek, D. Piontek, I. Wittenberg, A. Heßmer, K. Kraywinkel, C. Spix, and R. Pritzkuleit. 2023. Population-based clinical cancer registration in Germany. Cancers 15(15).
Kim, E., S. M. Rubinstein, K. T. Nead, A. P. Wojcieszynski, P. E. Gabriel, and J. L. Warner. 2019. The evolving use of electronic health records (EHR) for research. Seminars in Radiation Oncology 29(4):354–361.
Klora, M., P. Neuser, P. Morakis, S.-Z. Kim-Wanner, T. Brand, and A. Katalinic. 2024. Comprehensive oncological quality assurance by state cancer registries using the example of lung and cervical carcinomas. Die Onkologie 30:296–303.
Kohler, B. A., E. Ward, B. J. McCarthy, M. J. Schymura, L. A. Ries, C. Eheman, A. Jemal, R. N. Anderson, U. A. Ajani, and B. K. Edwards. 2011. Annual report to the nation on the status of cancer, 1975-2007, featuring tumors of the brain and other nervous system. Journal of the National Cancer Institute 103(9):714–736.
Kuderer, N. M., T. K. Choueiri, D. P. Shah, Y. Shyr, S. M. Rubinstein, D. R. Rivera, S. Shete, C. Y. Hsu, A. Desai, G. de Lima Lopes, Jr., P. Grivas, C. A. Painter, S. Peters, M. A. Thompson, Z. Bakouny, G. Batist, T. Bekaii-Saab, M. A. Bilen, N. Bouganim, M. B. Larroya, D. Castellano, S. A. Del Prete, D. B. Doroshow, P. C. Egan, A. Elkrief, D. Farmakiotis, D. Flora, M. D. Galsky, M. J. Glover, E. A. Griffiths, A. P. Gulati, S. Gupta, N. Hafez, T. R. Halfdanarson, J. E. Hawley, E. Hsu, A. Kasi, A. R. Khaki, C. A. Lemmon, C. Lewis, B. Logan, T. Masters, R. R. McKay, R. A. Mesa, A. K. Morgans, M. F. Mulcahy, O. A. Panagiotou, P. Peddi, N. A. Pennell, K. Reynolds, L. R. Rosen, R. Rosovsky, M. Salazar, A. Schmidt, S. A. Shah, J. A. Shaya, J. Steinharter, K. E. Stockerl-Goldstein, S. Subbiah, D. C. Vinh, F. H. Wehbe, L. B. Weissmann, J. T. Wu, E. Wulff-Burchfield, Z. Xie, A. Yeh, P. P. Yu, A. Y. Zhou, L. Zubiri, S. Mishra, G. H. Lyman, B. I. Rini, and J. L. Warner. 2020. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 395(10241):1907–1918.
Lee, H., and G. K. Singh. 2022. Disparities in all-cancer and lung cancer survival by social, behavioral, and health status characteristics in the United States: A longitudinal followup of the 1997–2015 National Health Interview Survey—National Death Index Record Linkage study. Journal of Cancer Prevention 27(2):89–100.
Lewis, C., C. Horstman, D, Blumenthal, and M. K. Abrams. 2023. Value-Based Care: What It Is, and Why It’s Needed. https://www.commonwealthfund.org/publications/explainer/2023/feb/value-based-care-what-it-is-why-its-needed (accessed October 8, 2024).
Mendez, J. S., L. Liu, J. P. Lewinger, L. Fejerman, and M. C. Stern. 2024. Abstract a008: Proof-of-concept: A machine learning classifier to impute nativity status in Hispanic/Latino/a/x cancer patients in the California cancer registry. Cancer Epidemiology, Biomarkers & Prevention 33(9_Supplement):A008.
Miller, T. P., Y. Li, A. J. Masino, E. Vallee, E. Burrows, M. Ramos, T. A. Alonzo, R. Gerbing, S. M. Castellino, D. S. Hawkins, T. L. Lash, R. Aplenc, and R. W. Grundmeier. 2022. Automated ascertainment of typhlitis from the electronic health record. JCO Clinical Cancer Informatics 6:e2200081.
Montealegre, J. R., R. Zhou, E. S. Amirian, and M. E. Scheurer. 2014. Uncovering nativity disparities in cancer patterns: Multiple imputation strategy to handle missing nativity data in the surveillance, epidemiology, and end results data file. Cancer 120(8):1203–1211.
NAACCR (North American Association of Central Cancer Registries). 2024. About NAACCR. https://www.naaccr.org/about-naaccr (accessed October 15, 2024).
NCCDPHP (National Center for Chronic Disease Prevention and Health Promotion). 2024. About the National Program of Cancer Registries. https://www.cdc.gov/national-program-cancer-registries/about/index.html (accessed October 13, 2024).
NCRA (National Cancer Registrars Association). 2024a. NCRA and Our Work. https://www.ncra-usa.org/About (accessed October 24, 2024).
NCRA. 2024b. What Is NCRA? https://www.ncra-usa.org/About/What-is-NCRA (accessed October 24, 2024).
Nogueira, L. M., F. P. May, K. R. Yabroff, and R. L. Siegel. 2024. Racial disparities in receipt of guideline-concordant care for early-onset colorectal cancer in the United States. Journal of Clinical Oncology 42(12):1368–1377.
ONC (Office of the National Coordinator for Health Information Technology). 2019. State Health Information Exchange. https://www.healthit.gov/topic/onc-hitech-programs/state-health-information-exchange (accessed October 24, 2024).
OCM (Oncology Care Model) Evaluation Team. 2024. Evaluation of the Oncology Care Model: Final Report. https://www.cms.gov/priorities/innovation/data-and-reports/2024/ocm-final-eval-report-2024 (accessed October 24, 2024).
OneFlorida+ Clinical Research Network. 2024. Infrastructure: The Data Trust. https://oneflorida.sites.medinfo.ufl.edu/about-2/infrastructure/ (accessed October 9, 2024).
Palis, B. E., L. M. Janczewski, A. E. Browner, J. Cotler, L. Nogueira, L. C. Richardson, V. Benard, R. J. Wilson, N. Walker, R. M. McCabe, D. J. Boffa, and H. Nelson. 2024. The National Cancer Database conforms to the Standardized Framework for Registry and Data Quality. Annals of Surgical Oncology 31(9):5546–5559.
Penberthy, L., and S. Friedman. 2024. The SEER Program’s evolution: Supporting clinically meaningful population-level research. JNCI Monographs 2024(65):110–117.
Peng, C., X. Yang, A. Chen, K. E. Smith, N. PourNejatian, A. B. Costa, C. Martin, M. G. Flores, Y. Zhang, T. Magoc, G. Lipori, D. A. Mitchell, N. S. Ospina, M. M. Ahmed, W. R. Hogan, E. A. Shenkman, Y. Guo, J. Bian, and Y. Wu. 2023. A study of generative large language model for medical research and healthcare. NPJ Digital Medicine 6(1):210.
PCR (Pennsylvania Cancer Registry). 2024. Pennsylvania Cancer Registry. https://www.pa.gov/en/agencies/health/healthcare-and-public-health-professionals/cancer-registry.html (accessed October 15, 2024).
Petkov, V. I., J. S. Byun, K. C. Ward, N. C. Schussler, N. P. Archer, S. Bentler, J. A. Doherty, E. B. Durbin, S. T. Gershman, I. Cheng, T. Insaf, L. Gonsalves, B. Y. Hernandez, L. Koch, L. Liu, A. Monnereau, B. M. Morawski, S. M. Schwartz, A. Stroup, C. Wiggins, X.-C. Wu, S. Bonds, S. Negoita, and L. Penberthy. 2024. Reporting tumor genomic test results to SEER registries via linkages. JNCI Monographs 2024(65):168–179.
Robert Koch Institute. 2016. Cancer in Germany in 2011/2012, 10th ed. Berlin, Germany: Robert Koch Institute and the Association of Population-based Cancer Registries in Germany. https://edoc.rki.de/bitstream/handle/176904/5699/cancer_germany_2011_2012.pdf (accessed October 16, 2024).
Robert Koch Institute. 2019. Cancer in Germany in 2015/2016, 12th ed. Berlin, Germany: Robert Koch Institute and the Association of Population-based Cancer Registries in Germany. https://edoc.rki.de/bitstream/handle/176904/6012/krebs_in_deutschland_2015_2016.pdf?sequence=1 (accessed October 16, 2024).
Robinson, T. J., L. E. Wilson, P. K. Marcom, M. Troester, C. F. Lynch, B. Y. Hernandez, E. Parrilla, H. A. Brauer, and M. A. Dinan. 2021. Analysis of sociodemographic, clinical, and genomic factors associated with breast cancer mortality in the linked Surveillance, Epidemiology, and End Results and Medicare Database. JAMA Network Open 4(10):e2131020.
Sanchez, P., A. L. Van Dyke, V. I. Petkov, Y. Yuan, S. Bonds, C. Valenzuela, A. W. Tuan, R. Moravec, S. F. Altekruse, A. D. Singhi, K. M. Serdy, Y. Wu, R. D. Cress, J. A. Doherty, L. Mueller, B. Y. Hernandez, C. F. Lynch, T. C. Tucker, X.-C. Wu, L. Matrisian, and L. Penberthy. 2024. NCI SEER-linked virtual tissue repository pilot. JNCI Monographs 2024(65):180–190.
Schmidt, A. L., C. Labaki, C. Y. Hsu, Z. Bakouny, N. Balanchivadze, S. A. Berg, S. Blau, A. Daher, T. El Zarif, C. R. Friese, E. A. Griffiths, J. E. Hawley, B. Hayes-Lattin, V. Karivedu, T. Latif, B. H. Mavromatis, R. R. McKay, G. Nagaraj, R. H. Nguyen, O. A. Panagiotou, A. J. Portuguese, M. Puc, M. Santos Dutra, B. A. Schroeder, A. Thakkar, E. M. Wulff-Burchfield, S. Mishra, D. Farmakiotis, Y. Shyr, J. L. Warner, and T. K. Choueiri. 2022. COVID-19 vaccination and breakthrough infections in patients with cancer. Annals of Oncology 33(3):340–346.
Schroeder, M. C., X. Gao, I. Lizarraga, A. R. Kahl, and M. E. Charlton. 2022. The impact of Commission on Cancer accreditation status, hospital rurality and hospital size on quality measure performance rates. Annals of Surgical Oncology 29(4):2527–2536.
Schulz, S., C. Lange, K. Emrich, and C. Justenhoven. 2024. Quality indicators show higher fulfilment in centers certified by the German Cancer Society. Gesundheitswesen 10.1055/a-2312–6116.
SEER (Surveillance, Epidemiology, and End Results Program). 2024. About the SEER Registries. https://seer.cancer.gov/registries/ (accessed October 9, 2024).
Sharma, G. D., A. Yadav, and R. Chopra. 2020. Artificial intelligence and effective governance: A review, critique and research agenda. Sustainable Futures 2:100004.
Shulman, L. N., B. E. Palis, R. McCabe, K. Mallin, A. Loomis, D. Winchester, D. McKellar. 2018. Survival as a quality metric of cancer care: Use of the National Cancer Data Base to assess hospital performance. JCO Oncology Practice 14(1):e59–e72.
Shulman, L. N., A. E. Browner, B. E. Palis, K. Mallin, S. Kakade, N. Carp, R. McCabe, D. Winchester, S. L. Wong, and D. P. McKellar. 2019. Compliance with cancer quality measures over time and their association with survival outcomes: The Commission on Cancer’s experience with the quality measure requiring at least 12 regional lymph nodes to be removed and analyzed with colon cancer resections. Annals of Surgical Oncology 26(6):1613–1621.
Singh, A. P., E. P. Balogh, R. W. Carlson, M. M. Huizinga, B. A. Malin, A. Melamed, N. J. Meropol, E. D. Pisano, R. A. Winn, K. R. Yabroff, and L. N. Shulman. 2024. Re-envisioning electronic health records to optimize patient-centered cancer care, quality, surveillance, and research. JCO Oncology Practice:OP.24.00260.
Statistics Canada. 2024. Canadian Cancer Registry. https://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SDDS=3207 (accessed October 16, 2024).
Tevaarwerk, A. J., D. Karam, C. A. Gatten, E. S. Harlos, M. J. Maurer, K. V. Giridhar, T. C. Haddad, S. R. Alberts, S. J. Holton, A. Stockham, K. Leventakos, J. M. Hubbard, A. S. Mansfield, T. R. Halfdanarson, R. Chen, J. A. Jochum, A. S. Schwecke, R. A. Eiring, J. L. Carroll, I. B. Riaz, R. R. McWilliams, E. Galanis, and S. J. Mandrekar. 2024. Transforming the oncology data paradigm by creating, capturing, and retrieving structured cancer data at the point of care: A Mayo Clinic pilot. Cancer 10.1002/cncr.35304.
Torous, V. F., R. W. Simpson, J. P. Balani, A. S. Baras, M. A. Berman, G. G. Birdsong, G. A. Giannico, G. P. Paner, J. R. Pettus, Z. Sessions, S. J. Sirintrapun, J. R. Srigley, and S. Spencer. 2021. College of American Pathologists Cancer Protocols: From optimizing cancer patient care to facilitating interoperable reporting and downstream data use. JCO Clinical Cancer Informatics 47–55.
Tucker, T. C., E. B. Durbin, J. K. McDowell, and B. Huang. 2019. Unlocking the potential of population-based cancer registries. Cancer 125(21):3729–3737.
Turbow, S., J. R. Hollberg, and M. K. Ali. 2021. Electronic health record interoperability: How did we get here and how do we move forward? JAMA Health Forum 2(3):e210253.
Unger, J. M., E. Cook, E. Tai, and A. Bleyer. 2016. The role of clinical trial participation in cancer research: Barriers, evidence, and strategies. American Society of Clinical Oncology Educational Book 35:185–198.
Wells, B. J., K. M. Chagin, A. S. Nowacki, and M. W. Kattan. 2013. Strategies for handling missing data in electronic health record derived data. eGEMS 1(3):1035.
White, M. C., F. Babcock, N. S. Hayes, A. B. Mariotto, F. L. Wong, B. A. Kohler, and H. K. Weir. 2017. The history and use of cancer registry data by public health cancer control programs in the United States. Cancer 123 (Suppl 24):4969–4976.
Yang, X., A. Chen, N. PourNejatian, H. C. Shin, K. E. Smith, C. Parisien, C. Compas, C. Martin, A. B. Costa, M. G. Flores, Y. Zhang, T. Magoc, C. A. Harle, G. Lipori, D. A. Mitchell, W. R. Hogan, E. A. Shenkman, J. Bian, and W. Wu. 2022a. A large language model for electronic health records. npj Digital Medicine 5(1):194.
Yang, S., Y. T. Shih, J. Huo, H. J. Mehta, Y. Wu, R. G. Salloum, M. Alvarado, D. Zhang, D. Braithwaite, Y. Guo, and J. Bian. 2022b. Procedural complications associated with invasive diagnostic procedures after lung cancer screening with low-dose computed tomography. Lung Cancer 165:141–144.
This page intentionally left blank.

































































